Abstract
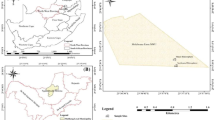
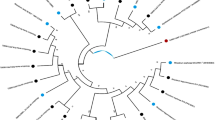
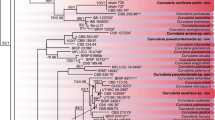
Kura clover (Trifolium ambiguum M.B.) is a perennial rhizomatous forage legume whose use is currently limited by difficulties in its establishment in part attributable to nodulation problems and very specific rhizobial requirements. A limited number of Kura clover-nodulating rhizobial strains are currently available and many have a limited effectiveness. In this study, 128 rhizobia were isolated from four sites in the center of origin of Kura clover (i.e., two in Azerbaijan, one in Armenia, and one in Northwest Iran) using the three ploidy levels of Kura clover (diploid, tetraploid, and hexaploid), red clover (Trifolium pratense L.), and white clover (Trifolium repens L.) plants as trap hosts. Rhizobia were fingerprinted using repetitive extragenic palindromic polymerase chain reaction (BOXA1R primer) and their genetic diversity was measured using the Shannon-Weaver diversity index. The nodulation specificity and phenotypic diversity of a subset of 13 isolates was determined. Genetic diversity among the 128 isolates was large and similar for rhizobia grouped according to their geographic origin or original host plant. Phenotypic diversity was significant; percentage of similarity among 13 isolates ranging between 38 and 92%. Nodulation specificity of the Kura clover-nodulating rhizobial isolates studied was less complex and not as clearly delineated as previously reported. Some strains originally isolated from Kura clover could effectively nodulate more than one ploidy level of Kura clover and even one or both of two other Trifolium species (i.e., red clover and white clover). Three strains formed effective nodules on both Kura clover and white clover; however, none promoted plant growth of both species to levels currently obtained with commercial inoculants when evaluated in a growth chamber. Rhizobial isolates that are highly effective with both species have yet to be identified.




Similar content being viewed by others
References
Applied Maths (2001) BioNumerics Manual, version 2.5. Applied Maths, Kortrijk
Balatti PA, Pueppke SG (1992) Identification of North American soybean lines forming nitrogen-fixing nodules with Rhizobium fredii USDA257. Can J Plant Sci 72:49–55
Beauregard M-S, Seguin P, Sheaffer CC, Graham PH (2003) Characterization and evaluation of North American Trifolium ambiguum-nodulating rhizobia. Biol Fertil Soils 38:311–318
Bernal G, Graham PH (2001) Diversity in the rhizobia associated with Phaseolus vulgaris L. in Ecuador, and comparisons with Mexican bean rhizobia. Can J Microbiol 47:526–534
Burton JC (1985) Rhizobium relationships. In: Taylor NL (ed) Clover science and technology. (Agronomy monograph no. 25) Soil Science Society of America, Madison, Wis.
Elliot RM, Lowther WL, Ronson CW (1998a) Interactions between rhizobia nodulating Trifolium ambiguum and Trifolium repens in the field. In: Elmerich C, Kondorosi A, Newton WE (eds). Biological nitrogen fixation for the 21st century. Kluwer, Dordrecht
Elliot RM, McIntyre HJ, Challis BC, Pryor HN, Lowther WL, Ronson CW (1998b) Rhizobium issues affecting the contribution of Caucasian clover to New Zealand pastoral agriculture. Proc NZ Grassl Assoc 60:207–211
Goodwin SB, Allard RW, Hardy SA, Webster RK (1992) Hierarchical structure of pathogenic variation among Rhynchosporium secalis populations in Idaho and Oregon. Can J Bot 70:810–817
Hely FW (1957) Symbiotic variation in T. ambiguum M. Bieb. with special reference to the nature of resistance. Aust J Biol Sci 10:1–16
Laberge G, Seguin P (2004) Le trèfle Kura: une légumineuse pour pâturages permanents. Cah Agric 13 (in press)
Lie TA (1978) Symbiotic specialisation in pea plants: the requirement of specific Rhizobium strains for peas from Afghanistan. Ann Appl Biol 88:462–465
Long SR (1996) Rhizobium symbiosis: nod factors in perspective. Plant Cell 8:1885–1898
Parker DT, Allen ON (1952) The nodulation status of Trifolium ambiguum. Proc Soil Sci Soc Am 16:350–353
Patrick HN, Lowther WL (1995) Influence of the number of rhizobia on the nodulation and establishment of Trifolium ambiguum. Soil Biol Biochem 27:717–720
Pryor HN, Lowther WL, McIntyre HJ, Ronson CW (1998) An inoculant Rhizobium strain for improved establishment and growth of hexaploid Caucasian clover (Trifolium ambiguum). NZ J Agric Res 41:179–189
Richardson J, Stead DE, Elphinstone JG, Coutts RHA (2002) Diversity of Bulkholderia isolates from woodland rhizosphere environments. J Appl Microbiol 93:616–630
Robinson AC (1969) Host selection for effective Rhizobium trifolii by red clover and subterranean clover in the field. Aust J Agric Res 20:1053–1060
SAS Institute (1985) SAS user’s guide, Statistics, 5th edn. SAS, Cary, N.C.
Seguin P, Graham PH, Sheaffer CC, Ehlke NJ, Ruselle MP (2001a) Genetic diversity of rhizobia nodulating Trifolium ambiguum in North America. Can J Microbiol 47:81–85
Seguin P, Sheaffer CC, Ehlke NJ, Russelle MP, Graham PH (2001b) Nitrogen fertilization and rhizobial inoculation effects on Kura clover growth. Agron J 93:1262–1269
Somasegaran P, Hoben HJ (1994) Handbook for rhizobia: methods in legume-Rhizobium technology. Springer, Berlin Heidelberg New York
Tesfaye M, Holl FB (1999) Rhizobium strains that nodulate Trifolium semipilosum Fres. are phylogenetically distinct. Plant Soil 207:147–154
Versalovic J, Schneider M, de Bruijn FJ, Lupski JR (1994) Genomic fingerprinting of bacteria using repetitive sequence-based polymerase chain reaction. Met Mol Cell Biol 5:25–40
Vincent JM (1970) A manual for the practical study of the root-nodule bacteria. IBP handbook no. 15. Blackwell, Oxford
Zhang X, Harper R, Karsisto M, Lindström K (1991) Diversity of Rhizobium bacteria isolated from the root nodules of leguminous trees. Int J Syst Bacteriol 41:104–113
Zhu Y, Sheaffer CC, Vance CP, Graham PH, Russelle MP, Montealegre C (1998) Inoculation and nitrogen affect herbage and symbiotic properties of annual Medicago species. Agron J 90:781–786
Zorin M, Dear BS, Hely FW (1976) Young plant vigour and nodulation studies in diploid forms of Trifolium ambiguum Bieb. Aust CSIRO Div Plant Ind Field Stn Res 15:35–40
Acknowledgements
The authors thank V. Alizade (Institute of Botany, Baku, Azerbaijan), E. Gabrielian (Institute of Botany, Yerevan, Armenia), N. Karimian (Nashtarud, Iran), and Y. Mohammadi (University of Ardabil, Ardabil, Iran), for supplying soil samples used for bacterial isolations; and N.L. Taylor (University of Kentucky) for supplying seeds of diploid and tetraploid Kura clover. This research was supported by research grants awarded to P. S. by the Natural Sciences and Engineering Research Council of Canada and the Research Development Fund of McGill University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Beauregard, MS., Zheng, W. & Seguin, P. Diversity of Trifolium ambiguum—nodulating rhizobia from the lower Caucasus. Biol Fertil Soils 40, 128–135 (2004). https://doi.org/10.1007/s00374-004-0752-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-004-0752-4