Abstract
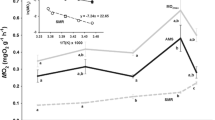
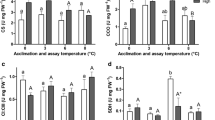
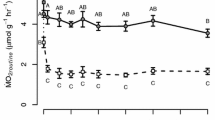
Seasonal changes in membrane composition and metabolic activity allow many temperate ectotherms to contend with changes in body temperature, but few studies have investigated whether the plasticity of these traits has diverged within a single species. Therefore, we studied the effects of thermal acclimation on the membrane fatty acid composition and the activities of cytochrome c oxidase (CCO) and citrate synthase (CS) in the skeletal muscle and liver of eastern newts from Maine and Florida. Newts were acclimated to either 6 °C or 28 °C for 12 weeks prior to experiments. Cold acclimation resulted in a lower saturated fatty acid (SFA) content in the muscle membranes of both populations. SFA content in liver was lower in cold compared to warm-acclimated newts from Florida, but acclimation did not affect SFA content in liver membranes of the Maine population. In liver, cold acclimation resulted in a higher monounsaturated fatty acid (MUFA) content in the Florida population and a higher polyunsaturated fatty acid (PUFA) content in the Maine population. Regardless of acclimation conditions, the muscle and liver membranes of the Maine population had higher SFA and PUFA contents compared to those of the Florida population. MUFA content of muscle and liver membranes was higher in the Florida population compared to the Maine population. The effect of acclimation on CCO and CS activity was tissue-specific. In muscle, CCO and CS activities were higher in cold compared to warm-acclimated newts in both populations, and CS and CCO activities were higher in the Maine compared to the Florida population. In liver, CCO and CS activity were unaffected by acclimation in the Florida population, but activity was lower in cold compared to warm-acclimated Maine newts. These results demonstrate that the phenotypic plasticity of these traits in response to seasonal change has diverged between northern and southern populations.


Similar content being viewed by others
References
Berner NJ, Bessay EP (2006) Correlation of seasonal acclimatization in metabolic enzyme activity with preferred body temperature in the eastern red spotted newt (Notophthalmus viridescens viridescens). Comp Biochem Physiol A 144:429–436
Berner NJ, Puckett RE (2010) Phenotypic flexibility and thermoregulatory behavior in the eastern red-spotted newt (Notophthalmus viridescens viridescens). J Exp Zool A 313:231–239
Berner NJ, Else PL, Hulbert AJ, Mantle BL, Cramp RL, Franklin CE (2009) Metabolic depression during aestivation does not involve remodeling of membrane fatty acids in two Australian frogs. J Comp Physiol B 179:857–866
Berner NJ, Bullock JR, Jerome WG, Marquand TH, Waldrup CL (2012) Tissue-specific acclimation of enzyme activity in the Eastern red spotted newt (Notophthalmus viridescens). Trends Comp Biochem Physiol 16:61–70
Budin I, de Rond T, Chen Y, Chan LJG, Petzold CJ, Keasling JD (2018) Viscous control of cellular respiration by membrane lipid composition. Science 362:1186–1189
Chevin LM, Hoffmann AA (2017) Evolution of phenotypic plasticity in extreme environments. Philos Trans R Soc B 372:2016038
Chung DJ, Sparagna GC, Chicco AJ, Schulte PM (2018) Patterns of mitochondrial membrane remodeling parallel functional adaptations to thermal stress. J Exp Biol 221:jeb174458. https://doi.org/10.1242/jeb.174458
Crocket EL (2008) The cold but not so hard fats in ectotherms: consequences of lipid restructuring on susceptibility of biological membranes to peroxidation, a review. J Comp Physiol 178:795–809
Crockett EL, Sidell BD (1990) Some pathways of energy metabolism are cold adapted in Antarctic fishes. Physiol Zool 63:472–488
Dhillon RS, Schulte PM (2011) Intraspecific variation in the thermal plasticity of mitochondria in killifish. J Exp Biol 214:3639–3648
Fangue NA, Richards JG, Schulte PM (2009) Do mitochondrial properties explain intraspecific variation in thermal tolerance? J Exp Biol 212:514–522
Feder ME, Lynch JF (1982) Effects of latitude, season, elevation, and microhabitat on field body temperatures of neotropical and temperate zone salamanders. Ecology 63:1657–1664
Ghalambor CK, Hoke KL, Ruell EW, Fischer EK, Reznick DN, Hughes KA (2015) Non-adaptive plasticity potentiates rapid adaptive evolution of gene expression in nature. Nature 525:372–375
Glanville EJ, Seebacher F (2006) Compensation for environmental change by complementary shifts of thermal sensitivity and thermoregulatory behavior in an ectotherm. J Exp Biol 209:4869–4877
Gray KT, Escobar AM, Schaeffer PJ, Mineo PM, Berner NJ (2016) Thermal acclimatization in overwintering Tadpoles of the Green Frog, Lithobates clamitans (Latreille 1801). J Exp Zool A 325:285–293
Guderley H (2004) Metabolic responses to low temperature in fish muscle. Biol Rev 79:409–427
Guderley H, St-Pierre J (2002) Going with the flow or life in the fast lane: contrasting mitochondrial responses to thermal change. J Exp Biol 205:2237–2249
Gvozdik L, Puky M, Sugerkova M (2007) Acclimation is beneficial at extreme test temperatures in the Danube crested newt, Triturus dobrogicus (Caudata, Salamandridae). Biol J Linnean Soc 90:627–636
Hazel JR (1979) Influence of thermal acclimation on membrane lipid composition of rainbow trout liver. Am J Physiol 236:R91–R101
Hazel JR (1995) Thermal adaptation in biological membranes: Is homeoviscous adaptation the explanation? Annu Rev Physiol 57:19–42
Holmstrup MA, Sorensen LI, Bindesbol AM, Hedlund A (2007) Cold acclimation and lipid composition in the earthworm Dendrobaena octaedra. Comp Biochem Physiol A 147:911–919
Hulbert AJ (2007) Membrane fatty acids as pacemakers of animal metabolism. Lipids 42:811–819
Hulbert AJ, Else PL (1999) Membranes as possible pacemakers of metabolism. J Theor Biol 199:257–274
Hulbert AJ, Pamplona R, Buffenstein R, Buttemer WA (2007) Life and death: metabolic rate, membrane composition, and life span of animals. Physiol Rev 87:1175–1213
Hulbert AJ, Kelly MA, Abbott SK (2014) Polyunsaturated fats, membrane lipids and animal longevity. J Comp Physiol B 184:149–166
Kraffe E, Marty Y, Guderley H (2007) Changes in mitochondrial oxidative capacities during thermal acclimation of rainbow trout Oncorhynchus mykiss: roles of membrane proteins, phospholipids, and their fatty acid compositions. J Exp Biol 210:149–165
Levis NA, Pfennig DW (2016) Evaluating ‘plasticity-first’ evolution in nature: key criteria and empirical approaches. TREE 31:563–574
Logue JA, De Vries AL, Fodor E, Cossins AR (2000) Lipid compositional correlates of temperature-adaptive interspecific differences in membrane physical structure. J Exp Biol 203:2105–2115
Mineo PM, Schaeffer PJ (2015a) The thermal plasticity of locomotor performance has diverged between northern and southern populations of the eastern newt (Notophthalmus viridescens). J Comp Physiol B 185:103–110
Mineo PM, Schaeffer PJ (2015b) Does the thermal plasticity of metabolic enzymes underlie thermal compensation of locomotor performance in the eastern newt (Notophthalmus viridescens)? J Exp Zool 232:52–59
Pamplona R, Portero-Otin M, Requena JR, Thorpe SR, Herrero A, Barja G (1999) A low degree of fatty acid unsaturation leads to lower lipid peroxidation and lipoxidation-derived protein modification in heart mitochondria of the longevous pigeon than in the short-lived rat. Mech Ageing Dev 106:283–296
Petranka JW (2010) Salamanders of the United States and Canada. Smithsonian Books, Washington, DC
Price ER, Sirsat TS, Sirsat SKG, Kang G, Keereetaweep J, Aziz M, Chapman KD, Dzialowski EM (2017) Thermal acclimation in American alligators: effects of temperature regime on growth rate, mitochondrial function, and membrane composition. J Therm Biol 68:45–54
Reynolds AM, Lee RE, Costanzo JP (2014) Membrane adaptation in phospholipids and cholesterol in the widely distributed, freeze-tolerant wood frog, Rana sylvatica. J Comp Physiol B 184:371–383
Rogers KD, Seebacher F, Thompson MB (2004) Biochemical acclimation of metabolic enzymes in response to lowered temperature in tadpoles of Limnodynastes peronii. Comp Biochem Physiol A 137:731–738
Rogers KD, Thompson MB, Seebacher F (2007) Beneficial acclimation: sex specific thermal acclimation of metabolic capacity in the striped marsh frog (Limnodynastes peronii). J Exp Biol 210:2932–2938
Rogowitz GL (1996) Evaluation of thermal acclimation and altitudinal variation of metabolism in a neotropical lizard (Anolis gundlachi). Copeia 1996:535–542
Rohr JR, Civitello DJ, Cohen JM, Roznik EA, Sinervo B, Dell AI (2018) The complex drivers of thermal acclimation and breadth in ectotherms. Ecol Lett 21:1425–1439
Seebacher F (2005) A review of thermoregulation and physiological performance in reptiles: what is the role of phenotypic flexibility? J Comp Physiol B 175:453–461
Seebacher F, Guderley H, Elsey RM, Trosclair PL (2003) Seasonal acclimatization of muscle metabolic enzymes in a reptile (Alligator mississippiensis). J Exp Biol 206:1993–1200
Turner N, Else PL, Hulbert AJ (2003) Docosahexaenoic acid (DHA) content of membranes determines molecular activity of the sodium pump: implications for disease states and metabolism. Naturwissenschaften 90:521–523
Wilson RS, Franklin CE (1999) Thermal acclimation of locomotor performance in tadpoles of the frog Limnodynastes peronii. J Comp Physiol B 169:445–451
Wilson RS, James RS, Johnston IA (2000) Thermal acclimation of locomotor performance in tadpoles and adults of the aquatic frog (Xenopus laevis). J Comp Physiol B 170:117–124
Wodtke E (1978) Lipid adaptation in liver mitochondrial membranes of carp acclimated to different environmental temperatures. Phospholipid composition, fatty acid pattern, and cholesterol content. Biochim Biophys Acta 529:280–291
Wodtke E (1981) Temperature adaptation of biological membranes. The effects of acclimation temperature on the unsaturation of the main neutral and charged phospholipids in mitochondrial membranes of the carp (Cyprinus carpio L.). Biochim Biophys Acta 640:698–709
Acknowledgements
We thank the Company of Biologists for providing a travel fellowship for Patrick Mineo to travel to Dr. Nancy J. Berner’s lab to measure membrane fatty acid composition for this project. We also thank Michael Oxendine for his assistance collecting newts and Keely Corder for her assistance with enzyme assays. Rick Wilson and Andrew McCullough from the Cathance River Preserve and Brunswick High School also deserve thanks for their assistance in locating and collecting newts in Maine. A. John Bailer, Jon P. Costanzo, Kathleen A. Killian, and Richard E. Lee, Jr. contributed constructive critiques of this manuscript. We also thank Rachael Morgan-Kiss for allowing use of her spectrophotometer. This work was supported by a Journal of Experimental Biology travel fellowship to PMM, the National Science Foundation [IOS:1120448 to NJB], and departmental funds to PJS and PMM.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by G. Heldmaier.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mineo, P.M., Waldrup, C., Berner, N.J. et al. Differential plasticity of membrane fatty acids in northern and southern populations of the eastern newt (Notophthalmus viridescens). J Comp Physiol B 189, 249–260 (2019). https://doi.org/10.1007/s00360-019-01203-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-019-01203-1