Abstract
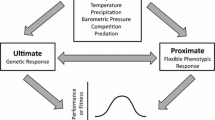
Life-history trade-offs result from allocation of limited energetic resources to particular traits at the expense of others. When resources are scarce, some traits will take priority over others in the degree of their expression. For example, the current reproduction may be sacrificed to enhance survival. Although intuitive from an evolutionary perspective, such priorities must be based on proximate mechanisms that respond to the current conditions. The hormone leptin serves as a signal of energy availability in vertebrates, and has been proposed as a mediator of energy allocation between reproduction and traits that enhance survival, such as the immune system. However, since leptin affects reproduction and immunity in a similar way, it remains unclear which takes priority when energy availability is low. Green anole lizards (Anolis carolinensis) with increased activity, via exercise training, have a marked decrease in immune function as well as reproduction, especially when calories are restricted. We hypothesized that endurance training and calorie restriction would lower immune and reproductive function due to energy limitation, and supplemental leptin would ‘rescue’ either immune function or reproduction (or both) due to the hormonal signal that energetic resources are available. We found that supplementary leptin rescued immune function in calorie-restricted, trained lizards, but reproduction was not rescued in males or females. This suggests that immune function and reproduction have different sensitivities to leptin in both sexes, or that reproduction is more energy limited and takes low priority even when the signal of energy availability is present.



Similar content being viewed by others
References
Abella V, Scotece M, Conde J, Pino J, Gonzalez-Gay MA, Gómez-Reino JJ, Mera A, Lago F, Gómez R, Gualillo O (2017) Leptin in the interplay of inflammation, metabolism and immune system disorders. Nat Rev Rheumatol 13:100–109
Angilletta MJ, Sears MW (2000) The metabolic cost of reproduction in an oviparous lizard. Funct Ecol 14:39–45
Baldelli R, Dieguez C, Casanueva FF (2002) The role of leptin in reproduction: experimental and clinical aspects. Ann Med 34:5–18
Carlton ED, Demas GE, French SS (2012) Leptin, a neuroendocrine mediator of immune responses, inflammation, and sickness behaviors. Horm Behav 62:272–279
Coccia E, Varricchio E, Putti R, Donizzetti I, Paolucci M (2011) Leptin in lizards: a new metabolic factor? In: Berhardt LV (ed) Advances in medicine and biology, vol 24. Nova Science Publishers, Hauppauge, pp 135–159
Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, Ohannesian JP, Marco CC, McKee LJ, Bauer TL, Caro JF (1996) Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med 334:292–295
Cotter SC, Simpson SJ, Raubenheimer D, Wilson K (2010) Macronutrient balance mediates trade-offs between immune function and life history traits. Funct Ecol 25:186–198
Demas GE, Sakaria S (2005) Leptin regulates energetic tradeoffs between body fat and humoural immunity. Proc R Soc B 272:1845–1850
Demas GE, Chefer V, Talan MI, Nelson RJ (1997) Metabolic costs of mounting an antigen-stimulated immune response in adult and aged C57BL/6J mice. Am J Physiol 42:R1631–R1637
Demas GE, Drazen DL, Nelson RJ (2003) Reductions in total body fat decrease humoral immunity. Proc R Soc B 270:905–911
Demas GE, Zysling DA, Beechler BR, Muehlenbein MP, French SS (2011) Beyond phytohaemagglutinin: assessing vertebrate immune function across ecological contexts. J Anim Ecol 80(4):710–730
Demas G, Grieves T, Chester E, French S (2012) The energetics of immunity: mechanisms mediating trade-offs in ecoimmunology. In: Demas G, Nelson RJ (eds) Ecoimmunology. Oxford University Press, Oxford, pp 259–296
Drazen DL, Demas GE, Nelson RJ (2001) Leptin effects on immune function and energy balance are photoperiod dependent in Siberian hamsters (Phodopus sungorus). Endocrinology 142:2768–2775
French SS, Dearing MD, Demas GE (2011) Leptin as a physiological mediator of energetic trade-offs in ecoimmunology: implications for disease. Integr Comp Biol 51:505–513
Hasselquist D, Nilsson JA (2012) Physiological mechanisms mediating costs of immune responses: what can we learn from studies of birds? Anim Behav 83:1303–1312
Hickey MS, Considine RV, Israel RG, Mahar TL, McCammon MR, Tyndall GL, Houmard JA, Caro JF (1996) Leptin is related to body fat content in male distance runners. Am J Physiol 271:E938–E940
Houmard JA, Cox JH, MacLean PS, Barakat HA (2000) Effect of short-term exercise training on leptin and insulin action. Metabolism 49:858–861
Husak JF, Lailvaux SP (2017) How do we measure the cost of whole-organism performance traits? Integr. Comp Biol 57:333–343
Husak JF, Irschick DJ, Meyers JJ, Lailvaux SP, Moore IT (2007) Hormones, sexual signals and performance of green anole lizards (Anolis carolinensis). Horm Behav 52:360–367
Husak JF, Irschick DJ, Henningsen JP, Kirkbride KS, Lailvaux SP, Moore IT (2009) Hormonal response of male green anole lizards (Anolis carolinensis) to GnRH challenge. J Exp Zool 311A:105–114
Husak JF, Keith AR, Wittry BN (2015) Making Olympic lizards: the effects of specialised exercise training on lizard performance. J Exp Biol 218:899–906
Husak JF, Ferguson HA, Lovern MB (2016) Trade-offs among locomotor performance, reproduction, and immunity in lizards. Funct Ecol 30:1665–1674
Husak JF, Roy JC, Lovern MB (2017) Exercise training reveals trade-offs between endurance performance and immune function, but does not affect growth, in juvenile lizards. J Exp Biol 220:1497–1502
Huyghe K, Husak JF, Herrel A, Tadic Z, Moore IT, Van Damme R, Vanhooydonck B (2009) Relationships between hormones, physiological performance and immunocompetence in a color-polymorphic lizard species, Podarcis melisellensis. Horm Behav 55:488–494
Ishigaki T, Koyama K, Tsujita J, Tanaka N, Hori S, Oku Y (2005) Plasma leptin levels of elite endurance runners after heavy endurance training. J Physiol Anthropol Appl Human Sci 24:573–578
Kimura M, Tateishi N, Shiota T, Yosie F, Yamauchi H, Suzuki M, Shibasaki T (2004) Long-term exercise down-regulates leptin receptor mRNA in the arcuate nucleus. Neuro Rep 15:713–716
Kraemer RR, Chu H, Castracane VD (2002) Leptin and exercise. Exp Biol Med 227:701–708
Lailvaux SP, Husak JF (2014) The life-history of whole-organism performance. Q Rev Biol 89:285–318
Lailvaux SP, Gilbert RL, Edwards JR (2012) A performance-based cost to honest signaling in male green anole lizards (Anolis carolinensis). Proc R Soc B 279:2841–2848
Lochmiller RL, Deerenberg C (2000) Trade-offs in evolutionary immunology: just what is the cost of immunity? Oikos 88:87–98
Londraville RL, Macotela Y, Duff RJ, Easterling MR, Liu Q, Crespi EJ (2014) Comparative endocrinology of leptin: assessing function in a phylogenetic context. Gen Comp Endocrinol 203:146–157
Lord GM, Matarese G, Howard JK, Baker RJ, Bloom SR, Lechler RI (1998) Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature 394:897–901
Maffei M, Halaas J, Ravussin E, Pratley RE, Lee GH, Zhang Y, Fei H, Kim S, Lallone R, Ranganathan S, Kern PA, Friedman JM (1995) Leptin levels in human and rodent: measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nat Med 1:1155–1161
Marler CA, Walsberg G, White ML, Moore M (1995) Increased energy expenditure due to increased territorial defense in male lizards after phenotypic manipulation. Behav Ecol Sociobiol 37:225–231
Martin TE (1987) Food as a limit on breeding birds: a life-history perspective. Annu Rev Ecol Syst 18:453–487
Martin LB, Han P, Kuhlman JR, Klasing KC, Wikelski M (2006) Phytohemagglutinin induced skin swelling in birds: histological support for a classic immunoecological technique. Funct Ecol 20:290–300
Moschos S, Chan JL, Mantzoros CS (2002) Leptin and reproduction: a review. Fertil Steril 77:433–444
Naylor C, Petri WA Jr (2016) Leptin regulation of immune responses. Trends Mol Med 22:88–98
Nelson RJ, Demas GE (1996) Seasonal changes in immune function. Q Rev Biol 71:511–548
Neuman-Lee LA, French SS (2014) Wound healing reduces stress-induced immune changes: evidence for immune prioritization in the side-blotched lizard. J Comp Physiol B 184:623–629
Niewiarowski PH, Balk ML, Londraville RL (2000) Phenotypic effects of leptin in an ectotherm: A new tool to study the evolution of life histories and endothermy? J Exp Biol 203:295–300
Nilsson J-Å, Råberg L (2001) The resting metabolic cost of egg laying and nestling feeding in great tits. Oecologia 128:187–192
Norgan NG (1997) The beneficial effects of body fat and adipose tissue in humans. Int J Obes Relat Metab Disord 21:738–746
Park HK, Ahima RS (2015) Physiology of leptin: energy homeostasis, neuroendocrine function and metabolism. Metabolism 64:24–34
Pérez-Pérez A, Vilariño-García T, Fernández-Riejos P, Martín-González J, Segura-Egea JJ, Sánchez-Margalet V (2017) Role of leptin as a link between metabolism and the immune system. Cytokine Growth Factor Rev 35:71–84
Ring BD, Scully S, Davis CR, Baker MB, Cullen MJ, Pelleymounter MA, Danilenko DM (2013) Systemically and topically administered leptin both accelerate wound healing in diabetic ob/ob mice. Endocrinology 141:446–449
Roberts ML, Buchanan KL, Evans MR (2004) Testing the immunocompetence handicap hypothesis: a review of the evidence. Anim Behav 68:227–239
Roff DA (1992) The evolution of life histories: theory and analysis. Chapman & Hall, New York
Sapolsky RM, Romero LM, Munck AU (2000) How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev 21:55–89
Schulte-Hostedde AI, Zinner B, Millar JS, Hickling GJ (2005) Restitution of mass-size residuals: validating body condition indices. Ecology 86:155–163
Smith KG, Hunt JL (2004) On the use of spleen mass as a measure of avian immune system strength. Oecologia 138:28–31
Smith GD, Neuman-Lee LA, Webb AC, Angilletta MJ Jr, DeNardo DF, French SS (2017) Metabolic responses to different immune challenges and varying resource availability in the side-blotched lizard (Uta stansburiana). J Comp Physiol B187:1173–1182
Speakman JR, Selman C (2003) Physical activity and resting metabolic rate. Proc Nutr Soc 62:621–634
Stearns SC (1992) The evolution of life histories. Oxford University Press, Oxford
Stearns SC (2000) Life history evolution: successes, limitations, and prospects. Naturwissenschaften 87:476–486
Swift DL, Johannsen NM, Lavie CJ, Earnest CP, Church TS (2014) The role of exercise and physical activity in weight loss and maintenance. Prog Cardiovasc Dis 56:441–447
Wabitsch M, Funcke JB, Lennerz B, Kuhnle-Krahl U, Lahr G, Debatin KM, Vatter P, Gierschik P, Moepps B, Fischer-Posovszky P (2015) Biologically inactive leptin and early-onset extreme obesity. N Engl J Med 372:48–54
Wadden TA, Considine RV, Foster GD, Anderson DA, Sarwer DB, Caro JS (1998) Short- and long-term changes in serum leptin in dieting obese women: effects of caloric restriction and weight loss. J Clin Endocrinol Metab 83:214–218
Wingfield JC, Farner DS (1975) The determination of five steroids in avian plasma by radioimmunoassay and competitive protein binding. Steroids 26:311–327
Woods SC, Seeley RJ, Porte D Jr, Schwartz MW (1998) Signals that regulate food intake and energy homeostasis. Science 280:1378–1383
Wotherspoon AD, Burgin S (2007) Lizard testes volume measurements: are they always underpinned by the correct assumptions? Eur J Anat 11:163–167
Yasari S, Wang D, Prud’homme D, Jankowski M, Gutkowska J, Lavoie JM (2009) Exercise training decreases plasma leptin levels and the expression of hepatic leptin receptor-a, -b, and, -e in rats. Mol Cell Biochem 324:13
Zamora-Camacho F, Reguera S, Rubiño-Hispán M, Moreno-Rueda G (2014) Eliciting an immune response reduces sprint speed in a lizard. Behav Ecol 26:115–120
Acknowledgements
We would like to thank J. FitzGerald, L. Henke, P. Sipe, and E. Magnuson for help with lizard husbandry, S. Lailvaux, K. Reardon, C. Rohlf, G. Solis, and E. Magnuson for comments on the previous drafts of the manuscript, and C. Wolfe for inspiration. AZW was partially funded by the Undergraduate Research Opportunities office at the University of St. Thomas.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by G. Heldmaier.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, A.Z., Husak, J.F. & Lovern, M. Leptin ameliorates the immunity, but not reproduction, trade-off with endurance in lizards. J Comp Physiol B 189, 261–269 (2019). https://doi.org/10.1007/s00360-019-01202-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-019-01202-2