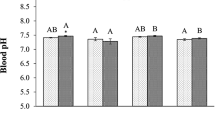
Abstract
To test the hypothesis of temperature-dependent deterioration of electrical excitability (TDEE) (Vornanen, J Exp Biol 219:1941–1952, 2016), the role of sodium (I Na) and calcium (I Ca) currents in heat tolerance of cardiac excitability was examined in a eurythermic fish, the roach (Rutilus rutilus). Densities of cardiac I Ca and I Na and their acute heat tolerance were measured in winter-acclimatized (WiR) and summer-acclimatized (SuR) fish maintained in the laboratory at 4 ± 1 and 18 ± 1 °C, respectively. A robust L-type Ca2+ current (I CaL), but no T-type Ca2+ current, was present in roach atrial and ventricular myocytes. Peak density of I CaL was smaller in atrial (− 1.97 ± 0.14 and − 1.75 ± 0.19 pA/pF for WiR and SuR, respectively) than ventricular myocytes (− 4.00 ± 0.59 and − 2.88 ± 0.47 pA/pF for WiR and SuR, respectively) (p < 0.05), but current density and heat tolerance of I CaL did not change between seasons in either cell type. In contrast to I Ca, marked differences appeared in I Na between WiR and SuR. I Na density was 38% higher in WiR than SuR atrial myocytes (− 80.03 ± 5.92 vs. − 49.77 ± 4.72 pA/pF; p < 0.05) and 48% higher in WiR than SuR ventricular myocytes (− 39.25 ± 3.06 vs. − 20.03 ± 1.79 pA/pF; p < 0.05). The winter increase in I Na density was associated with 55% (1.70 ± 0.27 vs. 0.77 ± 0.12) and 54% (1.08 ± 0.19 vs. 0.50 ± 0.10) up-regulation of the total Na+ channel (scn4 + scn5 + scn8) transcripts in atrium and ventricle, respectively (p < 0.05). Heat tolerance of atrial I Na was lower in WiR with a breakpoint temperature of 20.3 ± 1.2 °C than in SuR (23.8 ± 0.7 °C) (p < 0.05). The response of I Na to seasonal acclimatization conforms to the TDEE hypothesis. The lower heat tolerance of I Na in WiR is consistent with the lower heat tolerance of in vivo heart rate in WiR in comparison to SuR, but the match is not quantitatively perfect, suggesting that other factors in addition to I Na may be involved.






Similar content being viewed by others
Abbreviations
- AP:
-
Action potential
- CD:
-
Critical depolarization
- f H :
-
Heart rate
- HP:
-
Holding potential
- I Ca :
-
Calcium current
- I CaL :
-
L-type calcium current
- I CaT :
-
T-type calcium current
- I K1 :
-
Inward rectifier K+ current
- I Na :
-
Sodium current
- OCLTT:
-
Oxygen- and capacity-limited thermal tolerance hypothesis
- SuR:
-
Summer-acclimatized roach
- T BP :
-
Breakpoint temperature
- TDEE:
-
Temperature-dependent deterioration of electrical excitability hypothesis
- TP:
-
Threshold potential
- V 0.5 :
-
Voltage for half-maximal of activation or inactivation of I CaL
- WiR:
-
Winter-acclimatized roach
References
Abramochkin DV, Vornanen M (2016) Seasonal changes of cholinergic response in the atrium of Arctic navaga cod (Eleginus navaga). J Comp Physiol B 187:329–338
Badr A, El-Sayed MF, Vornanen M (2016) Effects of seasonal acclimatization on temperature-dependence of cardiac excitability in the roach, Rutilus rutilus. J Exp Biol 219:1495–1504
Badr A, Hassinen M, El-Sayed MF, Vornanen M (2017) Effects of seasonal acclimatization on action potentials and sarcolemmal K+ currents in roach (Rutilus rutilus) cardiac myocytes. Comp Biochem Physiol A 205:15–27
Bers DM (2002) Cardiac excitation–contraction coupling. Nature 415:198–205
Bers DM, Chen-Izu Y (2015) Sodium and calcium regulation in cardiac myocytes: from molecules to heart failure and arrhythmia. J Physiol 593:1327–1329
Bowler K (1981) Heat death and cellular heat injury. J Therm Biol 6:171–178
Clark TD, Sandblom E, Cox GK, Hinch SG, Farrell AP (2008) Circulatory limits to oxygen supply during an acute temperature increase in the Chinook salmon (Oncorhynchus tshawytscha). Am J Physiol 295:R1631-R1639
Cocking AW (1959) The effects of high temperatures on roach (Rutilus Rutilus): I. The effects of constant high temperatures. J Exp Biol 36:203–216
Cohen CJ, Fozzard HA, Sheu SS (1982) Increase in intracellular sodium ion activity during stimulation in mammalian cardiac muscle. Circ Res 50:651–662
Cossins AR, Prosser CL (1978) Evolutionary adaptation of membranes to temperature. Proc Natl Acad Sci USA 75:2040–2043
Farrell AP (2009) Environment, antecedents and climate change: lessons from the study of temperature physiology and river migration of salmonids. J Exp Biol 212:3771–3780
Fozzard HA (1977) Heart: excitation–contraction coupling. Ann Rev Physiol 39:201–220
Fozzard HA, Hanck DA (1996) Structure and function of voltage-dependent sodium channels: comparison of brain II and cardiac isoforms. Physiol Rev 76:887–926
Fry FEJ (1947) Effects of the environment on animal activity. Univ Tor Stud Biol Ser 55 68:1–62
Gollock MJ, Currie S, Petersen LH, Gamperl AK (2006) Cardiovascular and haematological responses of Atlantic cod (Gadus morhua) to acute temperature increase. J Exp Biol 209:2961–2970
Haverinen J, Vornanen M (2004) Temperature acclimation modifies Na+ current in fish cardiac myocytes. J Exp Biol 207:2823–2833
Haverinen J, Vornanen M (2006) Significance of Na+ current in the excitability of atrial and ventricular myocardium of the fish heart. J Exp Biol 209:549–557
Haverinen J, Abramochkin DV, Kamkin A, Vornanen M (2017) The maximum heart rate in brown trout (Salmo trutta fario) is not limited by firing rate of pacemaker cells. Am J Physiol 312:R165-R171
Kline RP, Morad M (1978) Potassium efflux in heart muscle during activity: extracellular accumulation and its implications. J Physiol 280:537–558
Kunze DL (1977) Rate-dependent changes in extracellular potassium in the rabbit atrium. Circ Res 41:122–127
Lagerspetz KYH (1987) Temperature effects on different organization levels in animals. Symp Soc Exp Biol 41:429–449
McDonald TF, Pelzer S, Trautwein W, Pelzer DJ (1994) Regulation and modulation of calcium channels in cardiac, skeletal, and smooth muscle cells. Physiol Rev 74:365–507
Pörtner HO (2001) Climate change and temperature-dependent biogeography: oxygen limitation of thermal tolerance in animals. Naturwissenschaften 88:137–146
Pörtner HO (2010) Oxygen- and capacity-limitation of thermal tolerance: a matrix for integrating climate-related stressor effects in marine ecosystems. J Exp Biol 213:881–893
Prosser CL, Nelson D (1981) The role of nervous systems in temperature adaptation of poikilotherms. Annu Rev Physiol 43:281–300
Rothschild LJ, Mancinelli RL (2001) Life in extreme environments. Nature 409:1092–1101
Sandblom E, Axelsson M (2007) Venous hemodynamic responses to acute temperature increase in the rainbow trout (Oncorhynchus mykiss). Am J Physiol 292:R2292–R2298
Shiels HA, Vornanen M, Farrell AP (2003) Acute temperature change modulates the response of I Ca to adrenergic stimulation in fish cardiomyocytes. Physiol Biochem Zool 76:816–824
Sinensky M (1974) Homeoviscous adaptation—a homeostatic process that regulates the viscosity of membrane lipids in Escerichia coli. Proc Natl Acad Sci USA 71:522–525
Somero GN (1995) Proteins and temperature. Annu Rev Physiol 57:43–68
Steinhausen MF, Sandblom E, Eliason EJ, Verhille C, Farrell AP (2008) The effect of acute temperature increases on the cardiorespiratory performance of resting and swimming sockeye salmon (Oncorhynchus nerka). J Exp Biol 211:3915–3926
Tiitu V, Vornanen M (2003) Ryanodine and dihydropyridine receptor binding in ventricular cardiac muscle of fish with different temperature preferences. J Comp Physiol B 173:285–291
Tikkanen E, Haverinen J, Egginton S, Hassinen M, Vornanen M (2017) Effects of prolonged anoxia on electrical activity of the heart in Crucian carp (Carassius carassius). J Exp Biol 220:445–454
Ushakov B (1964) Thermostability of cells and proteins of poikilotherms and its significance in speciation. Physiol Rev 44:518–560
Vornanen M (1997) Sarcolemmal Ca influx through L-type Ca channels in ventricular myocytes of a teleost fish. Am J Physiol 272:R1432-R1440
Vornanen M (1998) L-type Ca current in fish cardiac myocytes: effects of thermal acclimation and β-adrenergic stimulation. J Exp Biol 201:533–547
Vornanen M (2016) The temperature-dependence of electrical excitability of fish heart. J Exp Biol 219:1941–1952
Vornanen M, Hassinen M (2016) Zebrafish heart as a model for human cardiac electrophysiology. Channels (Austin) 10:101–110
Vornanen M, Paajanen V (2004) Seasonality of dihydropyridine receptor binding in the heart of an anoxia-tolerant vertebrate, the crucian carp (Carassius carassius L.). Am J Physiol 287:R1263–R1269
Vornanen M, Hassinen M, Koskinen H, Krasnov A (2005) Steady-state effects of temperature acclimation on the transcriptome of the rainbow trout heart. Am J Physiol 289:R1177–R1184
Vornanen M, Hassinen M, Haverinen J (2011) Tetrodotoxin sensitivity of the vertebrate cardiac Na+ current. Mar Drugs 9:2409–2422
Vornanen M, Haverinen J, Egginton S (2014) Acute heat tolerance of cardiac excitation in the brown trout (Salmo trutta fario). J Exp Biol 217:299–309
Wang DW, George AL, Bennett PB (1996) Comparison of heterologously expressed human cardiac and skeletal muscle sodium channels. Biophys J 70:238–245
Warren KS, Baker K, Fishman MC (2001) The slow mo mutation reduces pacemaker current and heart rate in adult zebrafish. Am J Physiol 281:H1711–H1719
Zhang S (2006) Isolation and characterization of I Kr in cardiac myocytes by Cs+ permeation. Am J Physiol 290:H1038–H1049
Acknowledgements
AB was supported by a personal grant from the Ministry of Higher Education (Cultural affairs and missions sector) of Egypt and Finnish Cultural foundation. A research Grant from the Academy of Finland (#14955) to MV covered the material costs of the study. We thank Kari Ratilainen for catching the fish and Anita Kervinen for assistance in taking care of the fishes and preparing the solutions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All experiments were authorized by the national animal experimental board in Finland (permission ESAVI/2832/04.10.07/2015).
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by G. Heldmaier.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Badr, A., Korajoki, H., Abu-Amra, ES. et al. Effects of seasonal acclimatization on thermal tolerance of inward currents in roach (Rutilus rutilus) cardiac myocytes. J Comp Physiol B 188, 255–269 (2018). https://doi.org/10.1007/s00360-017-1126-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-017-1126-1