Abstract
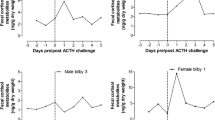
Knowledge of endocrine stress responses can be advantageous for understanding how animals respond to their environment. One tool in wildlife endocrinology is to measure the adrenocortical activity as a parameter of disturbance of animals. Fecal glucocorticoid metabolites (GCMs) provide a noninvasive assessment of adrenocortical activity. Using an adrenocorticotropic hormone (ACTH) challenge administered to 28 captive coyotes (Canis latrans), we measured the levels of plasma cortisol, and fecal cortisol and corticosterone metabolites (i.e., GCMs). Our goal was to determine the dose-response in the plasma and fecal samples following the injection and determine if there were effects of sex, age, and time of day. Specifically, animals were anesthetized for ~ 90 min with treatment animals intravenously injected with exogenous ACTH and control animals receiving saline. We collected blood samples prior to injection and at 4 different time points post-injection. We also collected fecal samples 2 days pre- and 2 days post-injection to measure fecal GCMs and determine if an endocrine stress response could be detected in fecal samples. We found a definite response in cortisol levels in the plasma for coyotes to the ACTH challenge. There was a response in fecal corticosterone 1 day post-injection, but the control males showed a similar response indicating a handling effect. Fecal cortisol levels did not indicate a response to the ACTH challenge, and were significantly lower than corticosterone concentrations. We also found significant sex, but not age or diurnal, differences in fecal GCMs. Radioimmunoassays for fecal corticosterone levels appeared to be a reliable indicator of physiological stress in coyotes.






Similar content being viewed by others
References
Aguilera G (2011) HPA axis responsiveness to stress: implications for healthy aging. Exp Gerontol 46:90–95
Arnemo JM, Caulkett N (2007) Stress. In: West G, Heard D, Caulkett N (eds) Zoo animal and wildlife immobilization and anesthesia. Blackwell Publishing Professional, Iowa, pp 102–110
Barja I, Silván G, Illera JC (2008) Relationships between sex and stress hormone levels in feces and marking behavior in a wild population of Iberian wolves (Canis lupus signatus). J Chem Ecol 34:697–701
Bauman JE, Hardin A (1998) Measurement of steroids in animal feces with commercially available RIA kits intended for use in human serum. J Clin Ligand Assay 21:83
Boonstra R (2004) Coping with changing northern environments: the role of the stress axis in birds and mammals. Integr Comp Biol 44:95–108
Brown JL, Kersey DC, Freeman EW, Wagener T (2010) Assessment of diurnal urinary cortisol excretion in Asian and African elephants using different endocrine methods. Zoo Biol 29:274–283
Carnes M, Kalin NH, Lent SJ, Barksdale CM, Brownfield MS (1988) Pulsatile ACTH secretion: variation with time of day and relationship to cortisol. Peptides 9:325–331
Cavigelli SA (1999) Behavioural patterns associated with faecal cortisol levels in free-ranging female ring-tailed lemurs, Lemur catta. Anim Behav 57:935–944
Cockrem JF (2013) Corticosterone responses and personality in birds: Individual variation and the ability to cope with environmental changes due to climate change. Gen Comp Endocrinol 190:156–163
Creel S (2005) Dominance, aggression, and glucocorticoid levels in social carnivores. J Mammal 86:255–264
Dalmau A, Ferret A, Chacon G, Manteca X (2007) Seasonal changes in fecal cortisol metabolites in Pyrean Chamois. J Wildl Manage 71:190–194
de Villiers MA, van Jaarsveld S, Meltzer DGA, Richardson PRK (1997) Social dynamics and the cortisol response to immobilization stress of the African wild dog, Lycaon pictus. Horm Behav 31:3–14
Dickmeis T (2009) Glucocorticoids and the circadian clock. J Endocrinol 200:3–22
Dloniak SM, French JA, Place NJ, Weldele ML, Glickman SE, Holekamp KE (2004) Non-invasive monitoring of fecal androgens in spotted hyenas (Crocuta crocuta). Gen Comp Endocrinol 135:51–61
French SS, Moore MC, Demas GE (2009) Ecological immunology: the organism in context. Integr Comp Biol 49:246–253
Galeandro L, Sieber-Ruckstuhl NS, Riond B, Hartnack S, Hofmann-Lehmann R, Reusch CE, Boretti FS (2014) Urinary corticoid concentrations measured by 5 different immunoassays and gas chromatography-mass spectrometry in healthy dogs and dogs with hypercortisolism at home and in the hospital. J Vet Internal Med 28:1433–1441
Gese EM (2001) Territorial defense by coyotes (Canis latrans) in Yellowstone National Park, Wyoming: who, how, where, when, and why. Can J Zool 79:980–987
Goymann W (2005) Noninvasive monitoring of hormones in bird droppings: physiological validation, sampling, extraction, sex differences, and the influence of diet on hormone metabolite levels. Ann NY Acad Sci 1046:35–53
Handa RJ, Burgess LH, Kerr JE, O’Keefe JA (1994) Gonadal steroid hormone receptors and sex differences in the hypothalamic-pituitary-adrenal axis. Horm Behav 28:464–476
Hergovich N, Singer E, Agneter E, Eichler HG, Graselli U, Simhandl C, Jilma B (2001) Comparison of the effects of ketamine and memantine on prolactin and cortisol release in men a randomized, double-blind, placebo- controlled trial. Neuropsychopharmacology 24:590–593
Hoon Son G, Chung S, Kim K (2011) The adrenal peripheral clock: Glucocorticoid and the circadian timing system. Front Neuroendocrinol 32:451–465
Huber S, Palme R, Arnold W (2003) Effects of season, sex, and sample collection on concentrations of fecal cortisol metabolites in red deer (Cervus elaphus). Gen Comp Endocrinol 130:48–54
Hulsman A, Dalerum F, Ganswindt A, Muenscher S, Bertschinger HJ, Paris M (2011) Non-invasive monitoring of glucocorticoid metabolites in Brown Hyaena (Hyaena brunnea) feces. Zoo Biol 30:451–458
Hunt KE, Wasser SK (2003) Effect of long-term preservation methods on fecal glucocorticoid concentrations of Grizzly bear and African elephant. Physiol Biochem Zool 76:918–928
Hunt KE, Trites AW, Wasser SK (2004) Validation of a fecal glucocorticoid assay for Stellar sea lions (Eumetopias jubatus). Physiol Behav 80:595–601
Keay JM, Singh J, Gaunt MC, Kaur T (2006) Fecal glucocorticoids and their metabolites as indicators of stress in various mammalian species: a literature review. J Zoo Wildl Med 37:234–244
Khalili-Mahani N, Martini CH, Olofsen E, Dahan A, Niesters M (2015) Effect of subanaesthetic ketamine on plasma and saliva cortisol secretion. Br J Anaesth 115:68–75
Laver PN, Ganswindt A, Ganswindt SB, Alexander KA (2012) Non-invasive monitoring of glucocorticoid metabolites in banded mongooses (Mungos mungo) in response to physiological and biological challenges. Gen Comp Endocrinol 179:178–183
Mashburn KL, Atkinson S (2004) Evaluation of adrenal function in serum and feces of Steller sea lions (Eumetopias jubatus): influences of molt, gender, sample storage, and age on glucocorticoid metabolism. Gen Comp Endocrinol. 136:371–381
Mateo JM, Cavigelli SA (2005) A validation of extraction methods for non-invasive sampling of glucocorticoids in free-living ground squirrels. Physiol Biochem Zool 78:1069–1084
McLeod PJ, Moger WH, Ryon J, Gadbois S, Fentress JC (1996) The relation between urinary cortisol levels and social behaviour in captive timber wolves. Can J Zool 74:209–216
Meaney MJ (2001) Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annu Rev Neurosci 24:1161–1192
Millspaugh JJ, Washburn BE (2004) Use of fecal glucocorticoid metabolite measures in conservation biology research: considerations for application and interpretation. Gen Comp Endocrinol 138:189–199
Möstl E, Palme R (2002) Hormones as indicators of stress. Domest Anim Endocrinol 23:67–74
Möstl E, Messmann S, Bagu E, Robia C, Palme R (1999) Measurement of glucocorticoid metabolite concentrations in faeces of domestic livestock. J Vet Med A Physiol Pathol Clin Med 46:621–631
Monfort SL, Mashburn KL, Brewer BA, Creel SR (1998) Evaluating adrenal activity in African Wild Dogs (Lycaon pictus) by fecal corticosteroid analysis. J Zoo Wildl Med 29:129–133
Neuman-Lee LA, Brodie ED Jr, Hansen T, Brodie ED III, French SS (2017) To stress or not to stress: physiological responses to tetrodotoxin in resistant gartersnakes vary by sex. Comp Biochem Physiol, Part A 209:34–40
Neuman-Lee LA, French SS (2014) Wound healing reduces stress-induced immune changes: evidence for immune prioritization in the side-blotched lizard. J Comp Physiol B 184:623–629
Neuman-Lee LA, French SS (2017) Endocrine-reproductive-immune interactions in female and male Galápagos marine iguanas. Hormones Behav 88:60–69
Nováková M, Palme R, Kutalová H, Janský L, Frynta D (2008) The effects of sex, age and commensal way of life on levels of fecal glucocorticoid metabolites in spiny mice (Acomys cahirinus). Phys Behav 95:187–193
Owen MA, Czekala NM, Swaisgood RR, Steinman K, Lindburg DG (2005) Seasonal and diurnal dynamics of glucocorticoids and behavior in giant pandas. Ursus 16:208–221
Rodrigues da Paz RC, Souza NP, Brown JL (2014) Evaluation of glucocorticoid faecal monitoring as a non-invasive assessment of stress in captive crab-eating fox (Cerdocyoun thous) after ACTH stimulation. J Steroids Horm Sci S12:008
Romero LM (2002) Seasonal changes in plasma glucocorticoid concentrations in free-living vertebrates. Gen Comp Endocrinol 128:1–24
Sands J, Creel S (2004) Social dominance, aggression and faecal glucocorticoid levels in a wild population of wolves, Canis lupus. Anim Behav 67:387–396
Santymire RM, Freeman EW, Lonsdorf EV, Heintz MR, Armstrong DM (2012) Using ACTH challenges to validate techniques for adrenocortical activity analysis in various African wildlife species. Int J Anim Vet Adv 4:99–108
Schatz S, Palme R (2001) Measurement of faecal cortisol metabolites in cats and dogs: a non-invasive method for evaluating adrenocortical function. Vet Res Commun 25:271–287
Schell CJ, Young JK, Lonsdorf EV, Santymire RM (2013) Anthropogenic and physiologically induced stress responses in captive coyotes. J Mammal 94:1131–1140
Sheriff MJ, Krebs CJ, Boonstra R (2010) Assessing stress in animal populations: Do fecal and plasma glucocorticoids tell the same story? Gen. Comp Endocrinol 166:614–619
Sheriff MJ, Dantzer B, Delehanty B, Palme R, Boonstra R (2011) Measuring stress in wildlife: techniques for quantifying glucocorticoids. Oecologia 166:869–887
Shideler SE, Savage A, Ortuno AM, Mooman EA, Lasley BL (1994) Monitoring female reproductive function by measurement of fecal estrogen and progesterone metabolites in the white–faced saki (Pithecia pithecia). Am J Primatol 32:95–108
Smith JE, Monclús R, Wantuck D, Florant GL, Blumstein DT (2012) Fecal glucocorticoid metabolites in wild yellow-bellied marmots: experimental validation, individual differences and ecological correlates. Gen Comp Endocrinol 178:417–426
Touma C, Palme R (2005) Measuring fecal glucocorticoid metabolites in mammals and birds: the importance of validation. Ann NY Acad Sci 1046:54–74
Touma C, Sachser N, Möstl E, Palme R (2003) Effects of sex and time of day on metabolism and excretion of corticosterone in urine and feces of mice. Gen Comp Endocrinol 130:267–278
Vasconcellos AS, Chelini MM, Palme R, Guimarães MABV, Oliveira CA, Ades C (2011) Comparison of two methods for glucocorticoid evaluation in maned wolves. Pesqui Vet Bras 31:79–83
Viljoen JJ, Ganswindt A, Palme R, Reynecke HC, Du Toit JT, Langbauer WR Jr (2008) Measurement of concentrations of faecal glucocorticoid metabolites in free-ranging African elephants within the Kruger National Park. Koedoe 50:18–21
von der Ohe CG, Servheen C (2002) Measuring stress in mammals using fecal glucocorticoids: opportunities and challenges. Wildl Soc Bull 30:1215–1225
von der Ohe CG, Wasser SK, Hunt KE, Servheen C (2004) Factors associated with fecal glucocorticoids in Alaskan brown bears (Ursus arctos horribilis). Physiol Biochem Zool 77:313–320
Wasser SK, Hunt KE, Brown JL, Cooper K, Crockett CM, Bechert U, Millspaugh JJ, Larson S, Monfort SL (2000) A generalized fecal glucocorticoid assay for use in a diverse array of nondomestic mammalian and avian species. Gen Comp Endocrinol 120:260–275
Wasser SK, Azkarate JC, Booth RK, Hayward L, Hunt K, Ayres K, Vynne C, Gobush K, Canales-Espinosa D, Rodríguez-Luna E (2010) Non-invasive measurement of thyroid hormone in feces of a diverse array of avian and mammalian species. Gen Comp Endocrinol 168:1–7
Young KM, Walker SL, Lanthier C, Waddell WT, Monfort SL, Brown JL (2004) Noninvasive monitoring of adrenocortical activity in carnivores by fecal glucocorticoid analyses. Gen Comp Endocrinol 137:148–165
Zeugswetter FK, Neffe F, Schwendenwein I, Tichy A, Möstl E (2013) Configuration of antibodies for assay of urinary cortisol in dogs influences analytic specificity. Domest Anim Endocrinol 45:98–104
Acknowledgements
We thank the USDA/APHIS/WS/National Wildlife Research Center, Predator Research facility for funding and use of the captive coyote colony. We also thank the staff at the Predator Research facility, especially S. Brummer, J. Schultz, and N. Floyd for helping with the care of the coyotes. We also thank B. Roberts for teaching us the extraction method, M. Stevenson for the help extracting the hormones from fecal samples, and J. Young for reviewing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable national and institutional guidelines for the care and use of animals were followed. Animal care, anesthesia, and handling procedures were reviewed and approved by the Institutional Animal Care and Use Committees (IACUC-QA 1809) at the USDA-National Wildlife Research Center and Utah State University.
Additional information
Communicated by G. Heldmaier.
Rights and permissions
About this article
Cite this article
Stevenson, E.T., Gese, E.M., Neuman-Lee, L.A. et al. Levels of plasma and fecal glucocorticoid metabolites following an ACTH challenge in male and female coyotes (Canis latrans). J Comp Physiol B 188, 345–358 (2018). https://doi.org/10.1007/s00360-017-1125-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-017-1125-2