Abstract
The energetic cost of immunity depends on many factors, including the type of challenge, the timing of the response, and the state of the animal. We measured changes in the standard metabolic rates of side-blotched lizards (Uta stansburiana Baird and Girard, 1852) in response to different immune challenges and nutritional states. In the first experiment, lizards were randomly assigned to one of four treatments: lipopolysaccharide (LPS) injection (to stimulate the response to a pathogen), cutaneous biopsy (as a proxy to a superficial wound), both injection and biopsy, or neither (control). Four and five days later, we measured the standard metabolic rates of the lizards. In response to healing a cutaneous wound, lizards reduced metabolic rate and lost body mass. Healing rate was also inversely related to weight loss, but LPS had no effect on body mass or metabolic rate. In the second experiment, a new set of lizards were randomly assigned to a high-food or low-food diet and administered a cutaneous biopsy. As in the first experiment, we observed a reduction in metabolic rate after wounding; moreover, this decrease was positively correlated with the rate of healing. We observed higher rates of metabolism in lizards that ate more food, but food consumption was unrelated to the decrease in metabolic rate following the biopsy. These experiments demonstrate the dynamic nature of the immune response in response to immune challenge and the state of the organism.






Similar content being viewed by others
References
Ahmed A, Baggott S, Maingon R, Hurd H (2002) The costs of mounting an immune response are reflected in the reproductive fitness of the mosquito Anopheles gambiae. Oikos 97(3):371–377
Ahtiainen J, Alatalo R, Kortet R, Rantala M (2005) A trade-off between sexual signalling and immune function in a natural population of the drumming wolf spider Hygrolycosa rubrofasciata. J Evol Biol 18(4):985–991
Alonso-Alvarez C, Tella JL (2001) Effects of experimental food restriction and body-mass changes on the avian T-cell-mediated immune response. Can J Zool 79(1):101–105
Andrén C, Nilson G (1983) Reproductive tactics in an island population of adders, Vipera berus (L.), with a fluctuating food resource. Amphib Reptil 4(1):63–79
Ardia DR, Parmentier HK, Vogel LA (2011) The role of constraints and limitation in driving individual variation in immune response. Funct Ecol 25(1):61–73
Barr DP, Cecil RL, Du Bois EF (1922) Clinical calorimetry XXXII: temperature regulation after the intravenous injection of proteose and typhoid vaccine. Arch Intern Med 29(5):608–634
Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M (2008) Growth factors and cytokines in wound healing. Wound Repair Regen 16(5):585–601
Bernheim HA, Kluger MJ (1976) Fever and antipyresis in the lizard Dipsosaurus dorsalis. Am J Physiol Leg Content 231(1):198–203
Cooper A, Fitzgeorge R, Baskerville A, Little R, Rothwell N (1989) Bacterial infection (Legionella pneumophila) stimulates fever, metabolic rate and brown adipose tissue activity in the Guinea pig. Life Sci 45(9):843–847
Cox CL, Peaden RT, Cox RM (2015) The metabolic cost of mounting an immune response in male brown anoles (Anolis sagrei). J Exp Zool Part A 323:689–695
Deen CM, Hutchison VH (2001) Effects of lipopolysaccharide and acclimation temperature on induced behavioral fever in juvenile Iguana iguana. J Therm Biol 26(1):55–63
Demas GE, Chefer V, Talan MI, Nelson RJ (1997) Metabolic costs of mounting an antigen-stimulated immune response in adult and aged C57BL/6 J mice. Am J Physiol Regul Integr Comp Physiol 273(5):R1631–R1637
Demas GE, Zysling DA, Beechler BR, Muehlenbein MP, French SS (2011) Beyond phytohaemagglutinin: assessing vertebrate immune function across ecological contexts. J Anim Ecol 80(4):710–730
Demas G, Greives T, Chester E, French S (2012) The energetics of immunity. In: Demas GE, Nelson RJ (eds) Ecoimmunology. Oxford University Press, Oxford, pp 259–296
Derting TL, Virk MK (2005) Positive effects of testosterone and immunochallenge on energy allocation to reproductive organs. J Comp Physiol B 175(8):543–556
do Amaral JPS, Marvin GA, Hutchison VH (2002) The influence of bacterial lipopolysaccharide on the thermoregulation of the box turtle Terrapene carolina. Physiol Biochem Zool 75(3):273–282
Fargallo JA, Laaksonen T, Pöyri V, Korpimäki E (2002) Inter-sexual differences in the immune response of Eurasian kestrel nestlings under food shortage. Ecol Lett 5(1):95–101
French SS, Neuman-Lee LA (2012) Improved ex vivo method for microbiocidal activity across vertebrate species. Biol Open 1(5):482–487
French SS, Matt KS, Moore MC (2006) The effects of stress on wound healing in male tree lizards (Urosaurus ornatus). Gen Comp Endocr 145(2):128–132
French SS, Johnston G, Moore M (2007a) Immune activity suppresses reproduction in food in food-limited female tree lizards Urosaurus ornatus. Funct Ecol 21(6):1115–1122
French SS, DeNardo DF, Moore MC (2007b) Trade-offs between the reproductive and immune systems: facultative responses to resources or obligate responses to reproduction? Am Nat 170(1):79–89
Huey RB, Pianka ER (1981) Ecological consequences of foraging mode. Ecology 62:991–999
Jacot A, Scheuber H, Brinkhof MW, Shaw K (2004) Costs of an induced immune response on sexual display and longevity in field crickets. Evol Int J Org Evol 58(10):2280–2286
Johnson R (2002) The concept of sickness behavior: a brief chronological account of four key discoveries. Vet Immunol Immunopathol 87(3):443–450
Klasing KC, Laurin DE, Peng RK, Fry DM (1987) Immunologically mediated growth depression in chicks: influence of feed intake, corticosterone and interleukin-1. J Nutr 117:1629–1637
Kluger MJ, Ringler DH, Anver MR (1975) Fever and survival. Science 188(4184):166–168
Kolbe JJ, Ehrenberger JC, Moniz HA, Angilletta MJ Jr (2014) Physiological variation among invasive populations of the brown anole (Anolis sagrei)*. Physiol Biochem Zool 87(1):92–104
Kristan DM, Hammond KA (2001) Parasite infection and caloric restriction induce physiological and morphological plasticity. Am J Physiol Regul Integr Comp Physiol 281(2):R502–R510
Laburn H, Mitchell D, Kenedi E, Louw G (1981) Pyrogens fail to produce fever in a cordylid lizard. Am J Physiol Regul Integr Comp Physiol 241(3):R198–R202
Lefcort H, Eiger SM (1993) Antipredatory behaviour of feverish tadpoles: implications for pathogen transmission. Behaviour 126(1):13–27
Liang Q-J, Lei Z, Qian C, Zheng W-H (2015) Effect of food restriction on the energy metabolism of the Chinese bulbul (Pycnonotus sinensis).
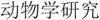 (Zool Res) 36 (2):79–87
(Zool Res) 36 (2):79–87Llewellyn D, Brown G, Thompson M, Shine R (2011) Behavioral responses to immune-system activation in an anuran (the cane toad, Bufo marinus): field and laboratory studies. Physiol Biochem Zool 84(1):77–86
Lochmiller RL, Deerenberg C (2000) Trade-offs in evolutionary immunology: just what is the cost of immunity? Oikos 88(1):87–98
López P, Gabirot M, Martín J (2009) Immune challenge affects sexual coloration of male Iberian wall lizards. J Exp Zool Part A Ecol Genet Physiol 311(2):96–104
Magnanou E, Fons R, Feliu C, Morand S (2006) Physiological responses of insular wild black rat (Rattus rattus) to natural infection by the digenean trematode Fasciola hepatica. Parasitol Res 99(1):97–101
Malvin MD, Kluger MJ (1979) Oxygen uptake in green iguana (Iguana iguana) injected with bacteria. J Thermal Biol 4(2):147–148
Martin LB II, Scheuerlein A, Wikelski M (2003) Immune activity elevates energy expenditure of house sparrows: a link between direct and indirect costs? Proc R Soc Lond Ser B Biol Sci 270(1511):153–158
Matson KD, Ricklefs RE, Klasing KC (2005) A hemolysis–hemagglutination assay for characterizing constitutive innate humoral immunity in wild and domestic birds. Dev Comp Immunol 29(3):275–286
Medzhitov R, Janeway J, Charles A (2000) Innate immunity. N Engl J Med 343:338–344
Merchant M, Fleury L, Rutherford R, Paulissen M (2008) Effects of bacterial lipopolysaccharide on thermoregulation in green anole lizards (Anolis carolinensis). Vet Immunol Immunopathol 125(1):176–181
Mills SC, Grapputo A, Jokinen I, Koskela E, Mappes T, Poikonen T (2010) Fitness trade-offs mediated by immunosuppression costs in a small mammal. Evol Int J Org Evol 64(1):166–179
Monagas W, Gatten R (1983) Behavioural fever in the turtles Terrapene carolina and Chrysemys picta. J Therm Biol 8(3):285–288
Moore MC (1986) Elevated testosterone levels during nonbreeding-season territoriality in a fall-breeding lizard, Sceloporus jarrovi. J Comp Physiol A 158(2):159–163
Murray M, Murray A (1979) Anorexia of infection as a mechanism of host defense. Am J Clin Nutr 32(3):593–596
Neuman-Lee LA, French SS (2014) Wound healing reduces stress-induced immune changes: evidence for immune prioritization in the side-blotched lizard. J Comp Physiol B 184(5):623–629
Neuman-Lee LA, Bobby Fokidis H, Spence AR, Van der Walt M, Smith GD, Durham S, French SS (2015) Food restriction and chronic stress alter energy use and affect immunity in an infrequent feeder. Funct Ecol 29(11):1453–1462
Norris K, Evans MR (2000) Ecological immunology: life history trade-offs and immune defense in birds. Behav Ecol 11(1):19–26
Nowell PC (1960) Phytohemagglutinin: an initiator of mitosis in cultures of normal human leukocytes. Cancer Res 20(4):462–466
Oro D, Genovart M, Tavecchia G, Fowler MS, Martínez-Abraín A (2013) Ecological and evolutionary implications of food subsidies from humans. Ecol Lett 16(12):1501–1514
Ots I, Kerimov AB, Ivankina EV, Ilyina TA, Hõrak P (2001) Immune challenge affects basal metabolic activity in wintering great tits. Proc R Soc Lond B Biol Sci 268(1472):1175–1181
Padgett DA, Glaser R (2003) How stress influences the immune response. Trends Immunol 24:444–448
Råberg L, Nilsson J, Ilmonen P, Stjernman M, Hasselquist D (2000) The cost of an immune response: vaccination reduces parental effort. Ecol Lett 3(5):382–386
Reznick D, Nunney L, Tessier A (2000) Big houses, big cars, superfleas and the costs of reproduction. Trends Ecol Evol 15(10):421–425
Roberts LA (1968) Oxygen consumption in the lizard Uta stansburiana. Ecology 49:809–819
Saino N, Calza S, Møller AP (1998) Effects of a dipteran ectoparasite on immune response and growth trade-offs in barn swallow, Hirundo rustica, nestlings. Oikos 81:217–228
Sanz JJ, Moreno J, Merino S, Tomás G (2004) A trade-off between two resource-demanding functions: post-nuptial moult and immunity during reproduction in male pied flycatchers. J Anim Ecol 73(3):441–447
Sherman E, Stephens A (1998) Fever and metabolic rate in the toad Bufo marinus. J Therm Biol 23(1):49–52
Soler JJ, de Neve L, Pérez–Contreras T, Soler M, Sorci G (2003) Trade-off between immunocompetence and growth in magpies: an experimental study. Proc R Soc Lond B Biol Sci 270(1512):241–248
Stebbins RC (2003) A field guide to western reptiles and amphibians. Peterson Field Guides, 3 edn. Houghton Mifflin Harcourt, Boston
Svensson E, Råberg L, Koch C, Hasselquist D (1998) Energetic stress, immunosuppression and the costs of an antibody response. Funct Ecol 12(6):912–919
Uller T, Isaksson C, Olsson M (2006) Immune challenge reduces reproductive output and growth in a lizard. Funct Ecol 20(5):873–879
Viney ME, Riley EM, Buchanan KL (2005) Optimal immune responses: immunocompetence revisited. Trends Ecol Evol 20(12):665–669
Waldschmidt S, Tracy CR (1983) Interactions between a lizard and its thermal environment: implications for sprint performance and space utilization in the lizard Uta stansburiana. Ecology 64:476–484
Werner S, Grose R (2003) Regulation of wound healing by growth factors and cytokines. Physiol Rev 83(3):835–870
White T (1978) The importance of a relative shortage of food in animal ecology. Oecologia 33(1):71–86
Wobeser GA (2006) Essentials of disease in wild animals. Blackwell Publishing Ltd., Oxford, UK
Zimmerman L, Vogel L, Bowden R (2010) Understanding the vertebrate immune system: insights from the reptilian perspective. J Exp Biol 213(5):661–671
Acknowledgements
We thank K. Moeller for laboratory assistance and P. Zani for help with the manuscript. S. Durham gave statistical and stylistic advice. We are also grateful for lizard-catching help from A. Berryman, A. Durso, D. Hunter, and M. Murphy.
Author contributions
GS and SF contributed to all aspects of this work. LN and AW contributed to performing experiments, assays, and manuscript revision. MA contributed to data analysis, manuscript revision, and provided technical expertise. DD contributed to experimental design, manuscript preparation, and provided technical expertise. These experiments were supported by the Utah State University Department of Biology and Ecology Center, as well as the National Science Foundation (IOS)-1350070.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No one involved with these experiments have any competing interests.
Additional information
Communicated by G. Heldmaier.
Rights and permissions
About this article
Cite this article
Smith, G.D., Neuman-Lee, L.A., Webb, A.C. et al. Metabolic responses to different immune challenges and varying resource availability in the side-blotched lizard (Uta stansburiana). J Comp Physiol B 187, 1173–1182 (2017). https://doi.org/10.1007/s00360-017-1095-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-017-1095-4




 (Zool Res) 36 (2):79–87
(Zool Res) 36 (2):79–87