Abstract
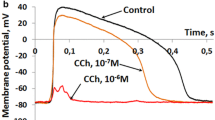
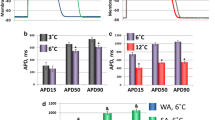
Fishes of north-temperate latitudes exhibit marked seasonal changes in electrical excitability of the heart partly as an outcome of temperature-dependent changes in the density of major K+ ion currents: delayed rectifiers (IKr, IKs) and background inward rectifier (IK1). In the arctic teleost, navaga cod (Eleginus navaga), IKr and IK1 are strongly up-regulated in winter. The current study tests the hypothesis that the ligand-gated K+ current, the acetylcholine-activated inward rectifier, IKACh, is also modified by seasonal acclimatization in atrial myocytes of navaga. In sinoatrial preparations of the summer-acclimatized (SA) navaga, 10−6 M carbamylcholine chloride (CCh) caused slowing of heart rate, shortening of atrial action potential (AP) duration and a drastic reduction of AP amplitude, eventually resulting in inexcitability. In winter-acclimatized (WA) atria CCh slowed HR and reduced AP duration, but reduction of AP amplitude was modest and never resulted in inexcitability. The difference in cholinergic response between SA and WA navaga is explained by seasonal changes in IKACh density. The peak density of IKACh, induced by 10−5 M CCh, at the common experimental temperature (+6 °C) was 0.97 ± 0.28 pA/pF in SA navaga but only 0.183 ± 0.013 pA/pF in WA navaga (a 5.3-fold difference, P < 0.05). At acclimatization temperatures of the fish IKACh density was 2.8 ± 0.50 (at +12 °C) and 0.11 ± 0.06 pA/pF (at +3 °C) (a 26-fold difference, P < 0.05) for SA and WA navaga, respectively. Thus, acclimatization to summer induces a drastic up-regulation of the atrial IKACh, which effectively shortens atrial AP. The reverse temperature compensation of the atrial IKACh may be advantageous in summer under variable water temperatures and oxygen concentrations by reducing workload of the heart.





Similar content being viewed by others
References
Abramochkin DV, Vornanen M (2015) Seasonal acclimatization of the cardiac potassium currents (IK1 and IKr) in an arctic marine teleost, the navaga cod (Eleginus navaga). J Comp Physiol B 185:883–890
Abramochkin DV, Kuzmin VS, Sukhova GS, Rosenshtraukh LV (2010) Cholinergic modulation of activation sequence in the atrial myocardium of non-mammalian vertebrates. Comp Biochem Physiol A 155:231–236
Abramochkin DV, Tapilina SV, Vornanen M (2014) A new potassium ion current induced by stimulation of M2 cholinoreceptors in fish atrial myocytes. J Exp Biol 217:1745–1751
Aho E, Vornanen M (2002) Effects of adenosine on the contractility of normoxic rainbow trout heart. J Comp Physiol B 172:217–225
Allen DG, Kentish JC (1985) The cellular basis of the length-tension relation in cardiac muscle. J Mol Cell Cardiol 17:821–840
Altimiras J, Axelsson M (2004) Intrinsic autoregulation of cardiac output in rainbow trout (Oncorhynchus mykiss) at different heart rates. J Exp Biol 207:195–201
Altimiras J, Aissaoui A, Tort L, Axelsson M (1997) Cholinergic and adrenergic tones in the control of heart rate in teleosts. How should they be calculated? Comp Biochem Physiol A 118:131–139
Badr A, El-Sayed MF, Vornanen M (2016) Effects of seasonal acclimatization on temperature-dependence of cardiac excitability in the roach, Rutilus rutilus. J Exp Biol 219:1495–1504
Belardinelli L, Isenberg G (1983) Isolated atrial myocytes: adenosine and acetylcholine increase potassium conductance. Am J Physiol 244:H734–H737
Bone Q, Marshall NB (1982) Biology of Fishes. Blackie & Son Limited, Glasgow London
Boyett M, Holden A, Kodama I, Suzuki R, Zhang H (1995) Atrial modulation of sinoatrial pacemaker rate. Chaos Soliton Fract 5:425–438
Dhein S, van Koppen CJ, Brodde O (2001) Muscarinic receptors in the Mammalian Heart. Pharmacol Res 44:161–182
Dobrzynski H, Marples DDR, Musa H, Yamanushi TT, Hendersonxyl Z, Takagishi Y, Honjo H, Kodama I, Boyett MR (2001) Distribution of the muscarinic K+ channel proteins Kir3.1 and Kir3.4 in the ventricle, atrium, and sinoatrial node of heart. J Histochem Cytochem 49:1221–1234
Driedzic WR, Gesser H (1994) Energy metabolism and contractility in ectothermic vertebrate hearts: Hypoxia, acidosis, and low temperature. Physiol Rev 74:221–258
Farrell AP (2007) Tribute to P. L. Lutz: a message from the heart–why hypoxic bradycardia in fishes? J Exp Biol 210:1715–1725
Farrell AP, MacLeod K, Driedzic WR (1982) The effects of preload, after load, and epinephrine on cardiac performance in the sea raven, Hemitripterus americanus. Can J Zool 60:3165–3171
Farrell AP, Small S, Graham M (1989) Effect of heart rate and hypoxia on the performance of a perfused trout heart. Can J Zool 67:274–280
Franklin CE, Axelsson M, Davison W (2001) Constancy and control of heart rate during an increase in temperature in the Antarctic fish Pagothenia borschgrevinki. Exp Biol 6:1–9
Fritsche R (1990) Effects of hypoxia on blood pressure and heart rate in three marine teleosts. Fish Physiol Biochem 8:85–92
Fritsche R, Nilsson S (1990) Autonomic nervous control of blood pressure and heart rate during hypoxia in the cod, Gadus morhua. J Comp Physiol B 160:287–292
Galli GL, Lipnick MS, Block BA (2009) Effect of thermal acclimation on action potentials and sarcolemmal K+ channels from Pacific bluefin tuna cardiomyocytes. Am J Physiol 297:R502–R509
Gamperl AK (2011) Integrated responses of the circulatory system to hypoxia. In: Farrell AP (ed) Encyclopedia of Fish Physiology. Academic Press, San Diego, pp 1221–1228
Hassinen M, Haverinen J, Vornanen M (2008) Electrophysiological properties and expression of the delayed rectifier potassium (ERG) channels in the heart of thermally acclimated rainbow trout. Am J Physiol 295:R297–R308
Hassinen M, Abramochkin DV, Vornanen M (2014) Seasonal acclimatization of the cardiac action potential in the Arctic navaga (Eleginus navaga, gadidae). J Comp Physiol B 184:319–327
Haverinen J, Vornanen M (2007) Temperature acclimation modifies sinoatrial pacemaker mechanism of the rainbow trout heart. Am J Physiol 292:R1023–R1032
Haverinen J, Vornanen M (2009) Responses of action potential and K+ currents to temperature acclimation in fish hearts: phylogeny or thermal preferences? Physiol Biochem Zool 82:468–482
Hibino H, Inanobe A, Furutani K, Murakami S, Findlay I, Kurachi Y (2010) Inwardly rectifying potassium channels: their structure, function and physiological role. Physiol Rev 90:291–366
Höglund L, Gesser H (1987) Electrical and mechanical activity in heart tissue of flounder and rainbow trout during acidosis. Comp Biochem Physiol 87A:543–546
Holeton GF, Randall DJ (1967) The effect of hypoxia upon the partial pressure of gases in the blood and water afferent and efferent to the gills of rainbow trout. J Exp Biol 46:317–327
Holmgren S (1977) Regulation of the heart of a teleost, Gadus morhua, by autonomic nerves and circulating catecholamines. Acta Physiol Scand 99:62–74
Huang TF (1973) The action potential of the myocardial cells of the golden carp. Jpn J Physiol 23:529–540
Keen AN, Fenna AJ, McConnell JC, Sherratt MJ, Gardner P, Shiels HA (2016) The dynamic nature of hypertrophic and fibrotic remodeling of the fish ventricle. Front Physiol 6:427
Klaiman JM, Fenna AJ, Shiels HA, Macri J, Gillis TE (2011) Cardiac remodeling in fish: strategies to maintain heart function during temperature change. PLoS One 6:e24464
Kodama I, Boyett MR, Suzuki R, Honjo H, Toyama J (1996) Regional differences in the response of the isolated sino-atrial node of the rabbit to vagal stimulation. J Physiol 495:785–801
Labat R, Serfaty A (1963) Electrocardiographie et vagotomie chez la Carpe commune. Bull Soc Hist Nat Toulouse 98:192–196
Lai NC, Graham JB, Dalton N, Shabetai R, Bhargava V (1998) Echocardiographic and hemodynamic determinations of the ventricular filling pattern in some teleost fishes. Physiol Zool 71:157–167
Laurent P, Holmgren S, Nilsson S (1983) Nervous and humoral control of the fish heart: structure and function. Comp Biochem Physiol A 76A:525–542
Levy MN (1971) Sympathetic-parasympathetic interactions in the heart. Circ Res 29:437–445
Lin TC, Hsieh JC, Lin CI (1995) Electromechanical effects of acetylcholine on the atrial tissues of the cultured tilapia (Oreochromis nilotica x O. aureus). Fish Physiol Biochem 14:449–457
Molina CE, Gesser H, Llach A, Tort L, Hove-Madsen L (2007) Modulation of membrane potential by an acetylcholine-activated potassium current in trout atrial myocytes. Am J Physiol 292:R388–R395
Nikolsky GV (1963) Ecology of Fishes. Academic Press
Precht H, Christophersen J, Hensel H (1955) Temperatur und Leben. Springer-Verlag, Berlin
Priede IG (1974) The effect of swimming activity and section of the vagus on heart rate in rainbow trout. J Exp Biol 60:305–319
Randall DJ (1982) The control of respiration and circulation in fish during exercise and hypoxia. J Exp Biol 100:275–288
Sandblom E, Axelsson M (2011) Autonomic control of circulation in fish: a comparative view. Auton Neurosci Basic 165:127–139
Sandblom E, Clark TD, Grans A, Ekstrom A, Brijs J, Sundstrom LF, Odelstrom A, Adill A, Aho T, Jutfelt F (2016) Physiological constraints to climate warming in fish follow principles of plastic floors and concrete ceilings. Nat Commun 7:11447
Seibert H (1979) Thermal adaptation of heart rate and its parasympathetic control in the European eel Anguilla anguilla. Comp Biochem Physiol 64C:275–278
Shiels HA, White E (2008) The Frank-Starling mechanism in vertebrate cardiac myocytes. J Exp Biol 211:2005–2013
Stecyk JA, Farrell AP (2006) Regulation of the cardiorespiratory system of common carp (Cyprinus carpio) during severe hypoxia at three seasonal acclimation temperatures. Physiol Biochem Zool 79:614–627
Sureau D, Lagardere JP, Pennec JP (1989) Heart rate and its cholinergic control in the sole (Solea vulgaris), acclimatized to different temperatures. Comp Biochem Physiol A 92A:49–51
Vornanen M (1997) Sarcolemmal Ca influx through L-type Ca channels in ventricular myocytes of a teleost fish. Am J Physiol 272:R1432–R1440
Vornanen M (2016) The temperature-dependence of electrical excitability of fish heart. J Exp Biol 219:1941–1952
Vornanen M, Ryökkynen A, Nurmi A (2002a) Temperature-dependent expression of sarcolemmal K+ currents in rainbow trout atrial and ventricular myocytes. Am J Physiol 282:R1191–R1199
Vornanen M, Shiels HA, Farrell AP (2002b) Plasticity of excitation-contraction coupling in fish cardiac myocytes. Comp Biochem Physiol A 132:827–846
Vornanen M, Hälinen M, Haverinen J (2010) Sinoatrial tissue of crucian carp heart has only negative contractile responses to autonomic agonists. BMC Physiol 10:10-6793-10-10
Wood CM, Shelton G (1980) The reflex control of heart rate and cardiac output in the rainbow trout: interactive influences of hypoxia, haemorrhage, and systemic vasomotor tone. J Exp Biol 87:271–284
Wood CM, Pieprzak P, Trott JN (1979) The influence of temperature and anemia on the adrenergic and cholinergic mechanisms controlling heart rate in the rainbow trout. Can J Zool 57:2440–2447
Acknowledgments
Authors thank the director of the White Sea Biological Station Dr. Alexander B. Tzetlin for general support of this study. Authors are also grateful to Valo V. Sivonen, Valentina P. Sivonen, Alexander L. Gvozdev and Dr. Maxim L. Lovat for capturing the fish. The study was supported by the Russian Foundation for Basic Research [14-04-01564 to D.V.A.] and the grant from The Academy of Finland to MV (Project No. 14955).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that they have no conflict of interest.
Ethical approval
All experiments conformed to the Guide for the Care and Use of Laboratory Animals published by the U.S. National Institutes of Health (NIH Publication No. 85–23, revised 1996)
Additional information
Communicated by H. V. Carey.
Rights and permissions
About this article
Cite this article
Abramochkin, D.V., Vornanen, M. Seasonal changes of cholinergic response in the atrium of Arctic navaga cod (Eleginus navaga). J Comp Physiol B 187, 329–338 (2017). https://doi.org/10.1007/s00360-016-1032-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-016-1032-y