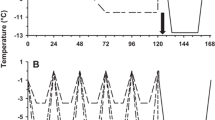
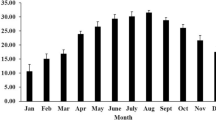
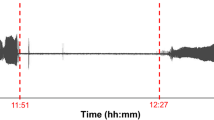
Abstract
The passage from shore to marine life is a critical step in the development of juvenile penguins and is characterized by a fuel selection towards lipid oxidation concomitant to an enhancement of lipid-induced thermogenesis. However, mechanisms of such thermogenic improvement at fledging remain undefined. We used two different groups of pre-fledging king penguins (Aptenodytes patagonicus) to investigate the specific contribution of cold exposure during water immersion to lipid metabolism. Terrestrial penguins that had never been immersed in cold water were compared with experimentally cold-water immersed juveniles. Experimentally immersed penguins underwent ten successive immersions at approximately 9–10 °C for 5 h over 3 weeks. We evaluated adaptive thermogenesis by measuring body temperature, metabolic rate and shivering activity in fully immersed penguins exposed to water temperatures ranging from 12 to 29 °C. Both never-immersed and experimentally immersed penguins were able to maintain their homeothermy in cold water, exhibiting similar thermogenic activity. In vivo, perfusion of lipid emulsion at thermoneutrality induced a twofold larger calorigenic response in experimentally immersed than in never-immersed birds. In vitro, the respiratory rates and the oxidative phosphorylation efficiency of isolated muscle mitochondria were not improved with cold-water immersions. The present study shows that acclimation to cold water only partially reproduced the fuel selection towards lipid oxidation that characterizes penguin acclimatization to marine life.





Similar content being viewed by others
References
Barré H, Rouanet JL (1983) Calorigenic effect of glucagon and catecholamines in king penguin chicks. Am J Physiol 244:758–763
Barré H, Roussel B (1986) Thermal and metabolic adaptation to first cold-water immersion in juvenile penguins. Am J Physiol 251:R456–R462
Barré H, Nedergaard J, Cannon B (1989a) Increased respiration in skeletal muscle mitochondria from cold-acclimated ducklings: uncoupling effects of free fatty acids. Comp Biochem Physiol 85B:343–348
Barré H, Berne G, Brebion P, Cohen-Adad F, Rouanet JL (1989b) Loose-coupled mitochondria in chronic glucagon-treated hyperthermic ducklings. Am J Physiol 256:R1192–R1199
Bedu E, Chainier F, Sibille B, Meister R, Dallevet G, Garin D, Duchamp C (2002) Increased lipogenesis in isolated hepatocytes from cold-acclimated ducklings. Am J Physiol 283:R1245–R1253
Bénistant C, Duchamp C, Cohen-Adad F, Rouanet JL, Barré H (1998) Increased in vitro fatty acid supply and cellular transport capacities in cold-acclimated ducklings (Cairina moschata). Am J Physiol 275:R683–R690
Bernard SF, Fayolle C, Robin JP, Groscolas R (2002) Glycerol and NEFA kinetics in long-term fasting king penguins: phase II versus phase III. J Exp Biol 205:2745–2754
Bevan RM, Woakes AJ, Butler PJ, Croxall JP (1995) Heart rate and oxygen consumption of exercising Gentoo penguins. Physiol Zool 68:855–877
Charrassin J, Bost C (2001) Utilisation of the oceanic habitat by king penguins over the annual cycle. Mar Ecol Prog Ser 221:285–298
Cherel Y, Durant JM, Lacroix A (2004) Plasma thyroid hormone pattern in king penguin chicks: a semi-altricial bird with an extended posthatching developmental period. Gen Comp Endocrinol 136:398–405
Conley KE (2016) Mitochondria to motion: optimizing oxidative phosphorylation to improve exercise performance. J Exp Biol 219:243–249
Dawson WR, Marsh RL, Yacoe ME (1983) Metabolic adjustments of small passerine birds for migration and cold. Am J Physiol 245:R755–R767
Duchamp C, Barré H (1993) Skeletal muscle as the major site of nonshivering thermogenesis in cold-acclimated ducklings. Am J Physiol 265:R1076–R1083
Duchamp C, Barré H, Delage D, Rouanet JL, Cohen-Adad F, Minaire Y (1989) Nonshivering thermogenesis and adaptation to fasting in king penguin chicks. Am J Physiol 257:R744–R751
Duchamp C, Barré H, Rouanet JL, Lanni A, Cohen-Adad F, Berne G, Brebion P (1991) Nonshivering thermogenesis in king penguin chicks. I. Role of skeletal muscle. Am J Physiol 261:R1438–R1445
Duchamp C, Cohen-Adad F, Rouanet JL, Barré H (1992) Histochemical arguments for muscular non-shivering thermogenesis in Muscovy ducklings. J Physiol 457:27–45
Duchamp C, Chatonnet J, Dittmar A, Barré H (1993) Increased role of skeletal muscle in the calorigenic response to glucagon of cold-acclimated ducklings. Am J Physiol 265:R1084–R1091
Duchamp C, Marmonier F, Denjean F, Lachuer J, Eldershaw TPD, Rouanet JL, Morales A, Meister R, Bénistant C, Roussel D, Barré H (1999) Regulatory, cellular and molecular aspects of avian muscle non-shivering thermogenesis. Ornis Fenn 76:151–165
Dumonteil E, Barré H, Rouanet JL, Diarra M, Bouvier J (1994) Dual core and shell temperature regulation during sea acclimatization in Gentoo penguins (Pygoscelis papua). Am J Physiol 266:R1319–R1326
Fahlman A, Schmidt A, Handrich Y, Woakes AJ, Butler PJ (2005) Metabolism and thermoregulation during fasting in king penguins, Aptenodytes patagonicus, in air and water. Am J Physiol 289:R670–R679
Froget G, Handrich Y, Le Maho Y, Rouanet JL, Woakes AJ, Butler PJ (2002) The heart rate/oxygen consumption relationship during cold exposure of the king penguin: a comparison with that during exercise. J Exp Biol 205:2511–2517
Froget G, Butler PJ, Woakes AJ, Fahlman A, Kuntz G, Le Maho Y, Handrich Y (2004) Heart rate and energetics of free-ranging king penguins (Aptenodytes patagonicus). J Exp Biol 207:3917–3926
Guglielmo CG (2010) Move that fatty acid: fuel selection and transport in migratory birds and bats. Integr Comp Biol 50:336–345
Handrich Y, Bevan RM, Charrassin JB, Butler PJ, Pütz K, Woakes AJ, Lage J, Le Maho Y (1997) Hypothermia in foraging king penguins. Nature 388:64–67
Hissa R (1988) Controlling mechanisms in avian temperature regulation: a review. Acta Physiol Scand 132:1–148
Humphries MM, Careau V (2011) Heat for nothing or activity for free? Evidence and implications of activity-thermoregulatory heat substitution. Integr Comp Biol 51:419–431
Kooyman GL, Davis RW, Croxall JP, Costa DP (1982) Diving depths and energy requirements of king penguins. Science 217:726–727
Mathieu-Costello O, Agey PJ, Quintana ES, Rousey K, Wu L, Bernstein MH (1998) Fiber capillarization and ultrastructure of pigeon pectoralis muscle after cold acclimation. J Exp Biol 201:3211–3220
Monternier PA, Marmillot V, Rouanet JL, Roussel D (2014) Mitochondrial phenotypic flexibility enhances energy savings during winter fast in king penguin chicks. J Exp Biol 217:2691–2697
Nagy KA, Kooyman GL, Ponganis PJ (2001) Energetic cost of foraging in free-diving emperor penguins. Physiol Biochem Zool 74:541–547
Péron C, Weimerskirch H, Bost CA (2012) Projected poleward shift of king penguins’ (Aptenodytes patagonicus) foraging range at the Crozet islands, southern Indian Ocean. Proc R Soc B 279:2515–2523
Pütz K, Cherel Y (2005) The diving behavior of brooding king penguins (Aptenodytes patagonicus) from the Falkland Islands: variation in dive profiles and synchronous underwater swimming provide new insights into their foraging strategies. Mar Biol 147:281–290
Rey B, Halsey L, Dolmazon V, Rouanet JL, Roussel D, Handrich Y, Butler P, Duchamp C (2008) Long-term fasting decreases mitochondrial avian UCP-mediated oxygen consumption in hypometabolic king penguins. Am J Physiol 295:R92–R100
Rey B, Roussel D, Romestaing C, Belouze M, Rouanet JL, Desplanches D, Sibille B, Servais S, Duchamp C (2010) Up-regulation of avian uncoupling protein in cold-acclimated and hyperthyroid ducklings prevents reactive oxygen species production by skeletal muscle mitochondria. BMC Physiol 10:5e
Roussel D, Rouanet JL, Duchamp C, Barré H (1998) Effects of cold acclimation and palmitate on energy coupling in duckling skeletal muscle mitochondria. FEBS Lett 439:258–262
Roussel D, Dumas JF, Simard G, Malthièry Y, Ritz P (2004) Kinetics and control of oxidative phosphorylation in rat liver mitochondria after dexamethasone treatment. Biochem J 382:491–499
Skulachev VP, Maslov SP (1960) The role of nonphosphorylating oxidation in temperature regulation. Biochemistry 25:1058–1064
Stahel CD, Nicol SC (1988) Ventilation and oxygen extraction in the little penguin (Eudyptula minor), at different temperatures in air and water. Respir Physiol 71:387–398
Swanson DL, Vézina F (2016) Environmental, ecological and mechanistic drivers of avian seasonal metabolic flexibility in response to cold winters. J Ornithol (in press)
Talbot DA, Duchamp C, Rey B, Hanuise N, Rouanet JL, Sibille B, Brand MD (2004) Uncoupling protein and ATP/ADP carrier increase mitochondrial proton conductance after cold adaptation of king penguins. J Physiol 558:123–135
Teulier L, Rouanet JL, Letexier D, Romestaing C, Belouze M, Rey B, Duchamp C, Roussel D (2010) Cold-acclimation-induced non-shivering thermogenesis in birds is associated with upregulation of avian UCP but not with innate uncoupling or altered ATP efficiency. J Exp Biol 213:2476–2482
Teulier L, Dégletagne C, Rey B, Tornos J, Keime C, de Dinechin M, Raccurt M, Rouanet JL, Roussel D, Duchamp C (2012) Selective upregulation of lipid metabolism in skeletal muscle of foraging juvenile king penguins: an integrative study. Proc R Soc B 279:2464–2472
Teulier L, Tornos J, Rouanet JL, Rey B, Roussel D (2013) Metabolic response to lipid infusion in fasting winter-acclimatized king penguin chicks (Aptenodytes patagonicus). Comp Biochem Physiol 165A:1
Toyomizu M, Ueda M, Sato S, Seki Y, Sato K, Akiba Y (2002) Cold-induced mitochondrial uncoupling and expression of chicken UCP and ANT mRNA in chicken skeletal muscle. FEBS Lett 529:313–318
Ueda M, Watanabe K, Sato K, Akiba Y, Toyomizu M (2005) Possible role for avPGC-1α in the control of expression of fiber type, along with avUCP and avANT mRNAs in the skeletal muscles of cold-exposed chickens. FEBS Lett 579:11–17
Vaillancourt E, Prud’Homme S, Haman F, Guglielmo C, Weber J (2005) Energetics of a long-distance migrant shorebird (Philomachus pugnax) during cold exposure and running. J Exp Biol 208:317–325
Weber JM (2009) The physiology of long-distance migration: extending the limits of endurance metabolism. J Exp Biol 212:593–597
Zhang Y, Eyster K, Liu JS, Swanson DL (2015) Cross-training in birds: cold and exercise training produce similar changes in maximal metabolic output, muscle masses and myostatin expression in house sparrows (Passer domesticus). J Exp Biol 218:2190–2200
Acknowledgments
The project was supported financially and logistically by the French Polar Institute (Institut Paul Emile Victor, IPEV—research program no. 131), and received a logistic support from the Terres Australes et Antarctiques Françaises.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by G. Heldmaier.
Rights and permissions
About this article
Cite this article
Teulier, L., Rey, B., Tornos, J. et al. Lipid-induced thermogenesis is up-regulated by the first cold-water immersions in juvenile penguins. J Comp Physiol B 186, 639–650 (2016). https://doi.org/10.1007/s00360-016-0975-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-016-0975-3