Abstract
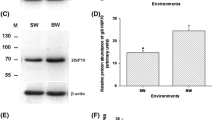
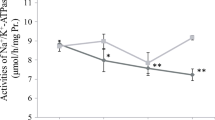
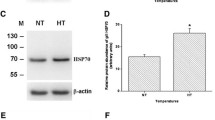
Salinity decreases are experienced by many marine elasmobranchs. To understand how these fishes cope with hyposmotic stress on a cellular level, we used the spiny dogfish shark (Squalus acanthias) as a model to test whether a reciprocal relationship exists between the cell’s two primary protein protection mechanisms, the chemical (e.g., trimethylamine oxide, TMAO) and molecular (e.g., heat shock protein 70, HSP70) chaperone systems. This relationship is interesting given that many elasmobranchs are expected to gain water and lose osmolytes, chemical chaperones, and ions as they osmoconform to new, lowered salinity. Dogfish were cannulated for repeated blood sampling and exposed to 70 % seawater (SW) for 48 h. These hyposmotic conditions had no effect on red blood cell (RBC) and white muscle TMAO concentrations, and did not result in HSP70 induction or signs of protein damage (i.e., increased ubiquitin), suggesting that TMAO levels were sufficiently protective in these tissues. However, in the gill, we observed a significant decrease in TMAO concentration and a significant induction of HSP70 as well as signs of protein damage. In the face of this cellular stress response, gill Na+/K+-ATPase (NKA) activity significantly increased during hyposmotic conditions, as expected. We suggest that this functional preservation in the gill is partly the result of HSP70 induction with lowered salinity. We conclude a reciprocal relationship between TMAO and HSP70 in the gills of dogfish as a result of in vivo hyposmotic stress. When osmotically induced protein damage surpasses the protective capacity of remaining TMAO, HSP70 is induced to preserve tissue and organismal function.







Similar content being viewed by others
References
Ballantyne JS, Fraser DI (2013) Euryhaline elasmobranchs. In: McCormick SD, Farrell AP, Brauner CJ (eds) Fish physiology: euryhaline fishes. Academic Press, Waltham, pp 126–179
Ballantyne JS, Robinson JW (2010) Freshwater elasmobranchs: a review of their physiology and biochemistry. J Comp Physiol 180B:475–493
Bedford JJ (1983) The effect of reduced salinity on tissue and plasma composition of the dogfish, Squalus acanthias. Comp Biochem Physiol 76A(1):81–84
Bentley PJ, Maetz J, Payan P (1976) A study of the unidirectional fluxes of Na and Cl across the gills of the dogfish Scyliorhinus canicula (Chondrichthyes). J Exp Biol 64:629–637
Branstetter S (1990) Early life-history implications of selected carcharhinoid and lamnoid sharks of the northwest Atlantic. In: Pratt HL Jr, Gruber SH, Taniuchi T (eds) Elasmobranchs as Living Resources: Advances in Biology, Ecology, Systematics and the Status of the Fisheries. NOAA Tech Rep 90, National Marine Fisheries Service, Silver Spring, MD, pp 17–28
Burger JW (1962) Further studies on the function of the rectal gland in the spiny dogfish. Physiol Zool 35:205–217
Burger JW, Tosteson DC (1966) Sodium influx and efflux in the spiny dogfish Squalus acanthias. Comp Biochem Physiol 19:649–653
Castro JI (1993) The shark nursery of Bulls Bay, South Carolina, with a review of the shark nurseries of the southeastern coast of the United States. Environ Biol Fish 38:37–48
Chan DKO, Phillips JG, Chester Jones I (1967) Studies on the electrolyte changes in the lip shark, Hemiscyllium plagiosum (Bennett), with special reference to hormonal influence on the rectal gland. Comp Biochem Physiol 23:185–198
Cooper AR, Morris S (1998) Osmotic and haematological response of the Port Jackson shark Heterodontus portusjacksoni and the common stingaree Trygonoptera testacea upon exposure to diluted seawater. Mar Biol 132:29–42
Currie S (2011) Heat shock proteins and temperature. In: Farrell AP (ed) Encyclopedia of fish physiology: from genome to environment, vol 3. Academic Press, San Diego, pp 1732–1737
Currie S, LeBlanc S, Watters MA, Gilmour KM (2010) Agonistic encounters and cellular angst: social interactions induce heat-shock proteins in juvenile salmonid fish. Proc R Soc Lond 277B:905–913
de Vlaming VL, Sage M (1973) Osmoregulation in the euryhaline elasmobranch, Dasyatis sabina. Comp Biochem Physiol 45A:31–44
DeBoeck G, Hattink J, Franklin NM, Bucking CP, Wood S, Walsh PJ, Wood CM (2007) Copper toxicity in the spiny dogfish (Squalus acanthias): urea loss contributes to osmoregulatory disturbance. Aquat Toxicol 84:133–142
Dowd WW, Harris BN, Cech JJ Jr, Kültz D (2010) Proteomic and physiological responses of leopard sharks (Triakis semifasciata) to salinity change. J Exp Biol 213:210–224
Duncan WP, Costa OTF, Araujo MLG, Fernandes MN (2009) Ionic regulation and Na+/K+-ATPase activity in gills and kidney of the freshwater stingray Paratrygon aiereba living in white and blackwaters in the Amazon Basin. J Fish Biol 74:956–960
Evans DH, Oikari A, Kormanik GA, Mansberger L (1982) Osmoregulation by the prenatal spiny dogfish, Squalus acanthias. J Exp Biol 101:295–305
Evans DH, Piermarini PM, Choe KP (2005) The multifunctional fish gill: dominant site of gas exchange, osmoregulation, acid-base regulation, and excretion of nitrogenous waste. Physiol Rev 85:97–177
Forster RP, Goldstein L (1976) Intracellular osmoregulatory role of amino acids and urea in marine elasmobranchs. Am J Physiol 230(4):925–931
Georgopoulos C, Welch WJ (1993) Role of the major heat shock proteins as molecular chaperones. Annu Rev Cell Biol 9:601–634
Guffey SC, Goss GG (2014) Time course of the acute response of the North Pacific spiny dogfish shark (Squalus suckleyi) to low salinity. Comp Biochem Physiol 171A:9–15
Hawkins AJS (1991) Protein turnover: a functional appraisal. Funct Ecol 5:222–233
Haywood GP (1975) A preliminary investigation into the roles played by the rectal gland and kidneys in the osmoregulation of the striped dogfish Poroderma africanum. J Exp Zool 193:167–176
Hazon N, Wells A, Pillans RD, Good JP, Anderson WG, Franklin CE (2003) Urea based osmoregulation and endocrine control in elasmobranch fish with special reference to euryhalinity. Comp Biochem Physiol 136B:685–700
Helfman G, Collette B, Facey D (1997) Chondrichthyes: sharks, skates, rays, and chimaeras. The Diversity of Fishes. Blackwell Science, Malden, pp 180–181
Hershko A, Ciechanover A (1998) The ubiquitin system. Annu Rev Biochem 67:425–479
Heupel MR, Carlson JK, Simpfendorfer CA (2007) Shark nursery areas: concepts, definition, characterization and assumptions. Mar Ecol Prog Ser 337:287–297
Hochachka PW, Somero GN (2002) Water-solute adaptations. In: Biochemical Adaptations, Oxford, New York, pp 217–289
Houlihan DF (1991) Protein turnover in ectotherms and its relationships to energetics. Adv Comp Env Physiol 7:1–43
Kajimura M, Walsh PJ, Mommsen TP, Wood CM (2006) The dogfish shark (Squalus acanthias) increases both hepatic and extrahepatic ornithine urea cycle enzyme activities for nitrogen conservation after feeding. Physiol Biochem Zool 79(3):602–613
Kajimura M, Walsh PJ, Wood CM (2008) The spiny dogfish Squalus acanthias L. maintains osmolyte balance during long-term starvation. J Fish Biol 72:656–670
Kolhatkar A, Robertson CE, Thistle ME, Gamperl AK, Currie S (2014) Coordination of chemical (trimethylamine oxide, TMAO) and molecular (HSP70) chaperone responses to heat stress in elasmobranch red blood cells. Physiol Biochem Zool 87(5):652–662
Koomoa D-LT, Musch MW, Vaz MacLean A, Goldstein L (2001) Volume-activated trimethylamine oxide efflux in red blood cells of spiny dogfish (Squalus acanthias). Am J Physiol Reg Integr Comp Physiol 281:R803–R810
Kültz D (1996) Plasticity and stressor specificity of osmotic and heat shock responses of Gillichthys mirabilis gill cells. Am J Physiol Cell Physiol 271(40):C1181–C1193
Kumar R (2009) Role of naturally occurring osmolytes in protein folding and stability. Arch Biochem Biophys 491:1–6
Laxson CJ, Condon NE, Drazen JC, Yancey PH (2011) Decreasing urea: trimethylamine N-oxide ratios with depth in Chondrichthyes: a physiological depth limit? Physiol Biochem Zool 84:494–505
LeBlanc S, Höglund E, Gilmour KM, Currie S (2011) Hormonal modulation of the heat shock response: insights from fish with divergent cortisol stress responses. Am J Physiol Regul Integr Comp Physiol 302:R184–R192
Marshall H, Field L, Afiadata A, Sepulveda C, Skomal G, Bernal D (2012) Hematological indicators of stress in longline-captured sharks. Comp Biochem Physiol 162A:121–129
Martin J, Langer T, Boteva R, Schramel A, Horwich AL (1991) Chaperonin-mediated protein folding at the surface of groEL through a “molten-globule”-like intermediate. Nature 325:36–45
McCormick SD (1993) Methods for nonlethal gill biopsy and measurement of Na+, K+-ATPase activity. Can J Fish Aquat Sci 50:656–658
Morgan JD, Wilson JM, Iwama GK (1997) Oxygen consumption and Na+, K+ ATPase activity of rectal gland and gill tissue of the spiny dogfish, Squalus acanthias. Can J Zool 75:820–825
Palmisano AN, Winton JR, Dickhoff WW (2000) Tissue-specific induction of hsp90 mRNA and plasma cortisol response in chinook salmon following heat shock, seawater challenge, and handling challenge. Mar Biotechnol 2:329–338
Pan F, Zarate JM, Tremblay GC, Bradley TM (2000) Cloning and characterization of salmon hsp90 cDNA: upregulation by thermal and hyperosmotic stress. J Exp Zool 287(3):199–212
Pang PKT, Griffith RW, Atz JW (1977) Osmoregulation in elasmobranchs. Am Zool 17(2):365–377
Piermarini PM, Evans DH (2000) Effects of environmental salinity on Na+/K+-ATPase in the gills and rectal gland of the euryhaline elasmobranch (Dasyatis sabina). J Exp Biol 203:2957–2966
Pillans RD, Good JP, Anderson WG, Hazon N, Franklin CE (2005) Freshwater to seawater acclimation of juvenile bull sharks (Carcharhinus leucas): plasma osmolytes and Na+/K+-ATPase activity in gill, rectal gland, kidney and intestine. J Comp Physiol 175B:37–44
Rahmatullah H, Boyde TRC (1980) Improvements in the determination of urea using diacetyl monoxide; methods with and without deproteinisation. Clin Chim Acta 107:3–9
Reilly BD, Cramp RL, Wilson JM, Campbell HA, Franklin CE (2011) Branchial osmoregulation in the euryhaline bull shark, Carcharhinus leucas: a molecular analysis of ion transporters. J Exp Biol 214:2883–2895
Sardella BA, Kültz D (2014) The physiological responses of green sturgeon (Acipenser medirostris) to potential global climate change stressors. Physiol Biochem Zool 87(3):456–463
Schmidt-Nielsen B, Truniger B, Rabinowitz L (1972) Sodium-linked urea transport by the renal tubule of the spiny dogfish Squalus acanthias. Comp Biochem Physiol 42A:13–25
Sheikh-Hamad D, García-Pérez A, Ferraris JD, Peters EM, Burg MB (1994) Induction of gene expression by heat shock versus osmotic stress. Am J Physiol Renal Fluid Electrolyte Physiol 267:F28–F38
Shuttleworth TJ, Thompson J, Munger RS, Wood CM (2006) A critical analysis of carbonic anhydrase function, respiratory gas exchange, and the acid-base control of secretion in the rectal gland of Squalus acanthias. J Exp Biol 209:4701–4716
Slee EA, Adrian C, Martin SJ (2001) Executioner caspase-3, -6, and -7 perform distinct, non-redundant roles during the demolition phase of apoptosis. J Biol Chem 276:7320–7326
Smith HW (1936) The retention and physiological role or urea in the Elasmobranchii. Biol Rev 11(1):49–82
Smith TR, Tremblay GC, Bradley TM (1999) Hsp70 and a 54 kDa protein (Osp54) are induced in salmon (Salmo salar) in response to hyperosmotic stress. J Exp Zool 284(3):286–298
Steele SL, Yancey PH, Wright PA (2005) The little skate Raja erinacea exhibits an extrahepatic ornithine urea cycle in the muscle and modulates nitrogen metabolism during low-salinity challenge. Physiol Biochem Zool 78(2):216–226
Stoothoff WH, Johnson GVW (2001) Hyperosmotic stress-induced apoptosis and tau phosphorylation in human neuroblastoma cells. J Neurosci Res 65:573–582
Tomanek L (2011) Environmental proteomics: changes in the proteome of marine organism in response to environmental stress, pollutants, infection, symbiosis, and development. Annu Rev Mar Sci 3:373–399
Treberg JR, Driedzic WR (2002) Elevated levels of trimethylamine oxide in deep-sea fish: evidence for synthesis and intertissue physiological importance. J Exp Zool 293:39–45
Treberg JR, Speers-Roesch B, Piermarini PM, Ip YK, Ballantyne JS, Driedzic WR (2006) The accumulation of methylamine counteracting solutes in elasmobranchs with differing levels of urea: a comparison of marine and freshwater species. J Exp Biol 209:860–870
Villalobos ARA, Renfro JL (2007) Trimethylamine oxide suppresses stress-induced alteration of organic anion transport in choroid plexus. J Exp Biol 210:541–552
Vyncke W (1970) Influence of biological and environmental factors on nitrogenous extractives of the spurdog, Squalus acanthias. Mar Biol 6:248–255
Wekell JC, Barnett H (1991) New method for analysis of trimethylamine oxide using ferrous sulfate and EDTA. J Food Sci 56:132–138
Wilson ED, McGuinn MR, Goldstein L (1999) Trimethylamine oxide transport across plasma membranes of elasmobranch erythrocytes. J Exp Zool 284:605–609
Wilson JM, Morgan JD, Vogl AW, Randall DJ (2002) Branchial mitochondria-rich cells in the dogfish Squalus acanthias. Comp Biochem Physiol 132A:365–374
Wood CM, Walsh PJ, Kajimura M, McClelland GB, Chew SF (2010) The influence of feeding and fasting on plasma metabolites in the dogfish shark (Squalus acanthias). Comp Biochem Physiol 155A:435–444
Wood CM, Liew HJ, De Boeck G, Walsh P (2013) A perfusion study of the handling of urea and urea analogues by the gills of the dogfish shark (Squalus acanthias). Peer J 1:e33
Wosnick N, Freire CA (2013) Some euryhalinity may be more common than expected in marine elasmobranchs: the example of the South American skate Zapteryx brevirostris (Elasmobranchii, Rajiformes, Rhinobatidae). Comp Biochem Physiol 166A:36–43
Wright PA, Wood CM (2015) Regulation of ions, acid-base and nitrogenous wastes. In: Shadwick R, Farrell AP, Brauner CJ (eds), Fish Physiology, vol 34B. Elsevier, San Diego, In press
Yancey PH (2005) Organic osmolytes as compatible, metabolic, and counteracting cytoprotectants in high osmolarity and other stresses. J Exp Biol 208:2819–2830
Yancey PH, Somero GN (1980) Methylamine osmoregulatory solutes of elasmobranch fishes counteract urea inhibition of enzymes. J Exp Zool 212:205–213
Yancey PH, Clark ME, Hand SC, Bowlus RD, Somero GN (1982) Living with water stress: evolution of osmolyte systems. Science 217:1214–1222
Acknowledgments
The authors wish to thank the staff of MDIBL for their assistance and support with this project, especially Michelle Bailey and her team for their help with animal care and acquisition and Wayne Sinclair for assistance with our experimental setup. We are also grateful to Natalie Donaher for her technical expertise, Dr. Diana Hamilton for her statistical prowess, Neal Callaghan for his invaluable help with the NKA assay, and Mike Lawrence for his assistance while at MDIBL and with the urea protocol. This work was funded by the Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery Grants (SC, PAW, TJM), by a Mount Allison University Carnegie Undergraduate Scholarship (RM), and by a Minogue Medical Inc. Undergraduate Research Award (RM).
Conflict of interest
The authors hereby disclose no conflict of interest, whether financial or otherwise. All procedures performed in studies involving animals were in accordance with the ethical standards of an approved IACUC at the MDIBL.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by H. V. Carey.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
MacLellan, R.J., Tunnah, L., Barnett, D. et al. Chaperone roles for TMAO and HSP70 during hyposmotic stress in the spiny dogfish shark (Squalus acanthias). J Comp Physiol B 185, 729–740 (2015). https://doi.org/10.1007/s00360-015-0916-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-015-0916-6