Abstract
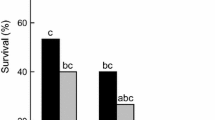
The cold tolerance of the Antarctic nematodes Scottnema lindsayae and Plectus murrayi was determined using material freshly isolated from the field. Both species could survive low temperatures but the survival of S. lindsayae was greater than that of P. murrayi. Field soil temperatures in late spring—early summer indicated a minimum temperature of −19.5 °C and a maximum cooling rate of 0.71 °C min−1. In P. murrayi grown in culture, there was no significant effect of acclimation, nor of the two culture media used, on survival after freezing but survival was greater if freezing was seeded at –1 °C than at lower temperatures. The freezing survival ability of P. murrayi is much less than that of Panagrolaimus davidi CB1, another Antarctic nematode. Cryomicroscopy indicates that P. murrayi can survive low temperatures by either cryoprotective dehydration or freezing tolerance, but that freezing tolerance is the dominant strategy. Measurable thermal hysteresis was detected only in highly concentrated extracts of the nematodes, indicating the presence of an antifreeze protein, but at the concentrations likely to be found in vivo, the major function of the ice active protein involved is probably recrystallization inhibition.






Similar content being viewed by others
Abbreviations
- ATW:
-
Artificial tap water
- BSS:
-
Balanced salt solution
- CL:
-
Confidence limits
- GLM:
-
General linear model
- \(n_{p}^{2}\) :
-
Partial eta squared
- S50 :
-
50 % survival temperature
- RMP:
-
Relative median potency
- Tmin :
-
Minimum temperature
References
Adhikari BN, Adams BJ (2011) Molecular analyses of desiccation survival in Antarctic nematodes. In: Perry RN, Wharton DA (eds) Molecular and physiological basis of nematode survival. CABI Publishing, Wallingford, pp 205–232
Adhikari BN, Wall DH, Adams BJ (2009) Desiccation survival in an Antarctic nematode: molecular analysis using expressed sequenced tags. BMC Genom 10:69. doi:10.1186/1471-2164-10-69
Adhikari BN, Wall DH, Adams BJ (2010) Effect of slow desiccation and freezing on gene transcription and stress survival of an Antarctic nematode. J Exp Biol 213:1803–1812
Ali F, Wharton DA (2014) Intracellular freezing in the infective juveniles of Steinernema feltiae: an entomopathogenic nematode. PLoS One 9(4):e94179. doi:10.1371/journal.pone.0094179
Brown IM, Wharton DA, Millar RB (2004) The influence of temperature on the life history of the Antarctic nematode Panagrolaimus davidi. Nematology 6:883–890
Caldwell JR (1981) The Signy Island terrestrial reference sites: XIII. Population dynamics of the nematode fauna. Br Antarc Surv Bull 54:33–46
Convey P, Worland MR (2000) Survival of freezing by free-living antarctic soil nematodes. CryoLetters 21:327–332
Duman JG (2001) Antifreeze and ice nucleator proteins in terrestrial arthropods. Ann Rev Physiol 63:327–357
Greenaway P (1970) Sodium regulation in the freshwater mollusc Limnaea stagnalis (L) (Gastropoda, Pulmonata). J Exp Biol 53:147–163
Holmstrup M (2014) The ins and outs of water dynamics in cold tolerant soil invertebrates. J Therm Biol 45:117–123
Hooper DJ (1986) Extraction of free-living stages from soil. In: Southey JF (ed) Laboratory methods for work with plant and soil nematodes. HMSO, London, pp 5–30
Jia Z, Davies PL (2002) Antifreeze proteins: an unusual receptor-ligand interaction. Trends Biochem Sci 27:101–106
Kawarasaki Y, Teets NM, Denlinger DL, Lee RE (2013) The protective effect of rapid cold-hardening develops more quickly in frozen versus supercooled larvae of the Antarctic midge, Belgica antarctica. J Exp Biol 216:3937–3945
Lewis SC, Dyal LA, Hilburn CF, Weitz S, Liau WS, LaMunyon CW, Denver DR (2009) Molecular evolution in Panagrolaimus nematodes: origins of parthenogenesis, hermaphroditism and the Antarctic species P. davidi. BMC Evol Biol 9:15
Nakagawa S, Cuthill IC (2007) Effect size, confidence interval and statistical significance: a practical guide for biologists. Biol Rev 82:591–605
Norusis MJ (1999) SPSS regression models 10.0. SPSS Inc, Chicago
Overhoff A, Freckman DW, Virginia RA (1993) Life cycle of the microbivorous Antarctic Dry Valley nematode Scottnema lindsayae (Timm 1971). Polar Biol 13:151–156
Pickup J (1990a) Seasonal variation in the cold hardiness of three species of free-living antarctic nematodes. Func Ecol 4:257–264
Pickup J (1990b) Seasonal variation in the cold-hardiness of a free-living predatory nematode, Coomansus gerlachei (Mononchidae). Polar Biol 10:307–315
Pickup J (1990c) Strategies of cold-hardiness in three species of antarctic dorylaimid nematodes. J Comp Physiol B 160:167–173
Piggott SJ, Perry RN, Wright DJ (2000) Hypo-osmotic regulation in entomopathogenic nematodes: Steinernema spp. and Heterorhabditis spp. Nematology 2:561–566
Ramløv H, Wharton DA, Wilson PW (1996) Recrystallization in a freezing tolerant Antarctic nematode, Panagrolaimus davidi, and an alpine weta, Hemideina maori (Orthoptera, Stenopelmatidae). Cryobiol 33:607–613
Raymond MR (2010) Cold-temperature Adaptation in Nematodes from the Victoria Land Coast, Antarctica. PhD thesis, University of Otago
Raymond MR, Wharton DA (2013) The ability of the Antarctic nematode Panagrolaimus davidi to survive intracellular freezing is dependent upon nutritional status. J Comp Physiol B 183:181–188
Raymond MR, Wharton DA, Marshall CJ (2013) Factors determining nematode distributions at Cape Hallett and Gondwana station, Antarctica. Antarc Sci 25:347–357. doi:10.1017/S0954102012001162
Raymond MR, Wharton DA, Marshall CJ (2014) Nematodes from the Victoria Land coast, Antarctica and comparisons with cultured Panagrolaimus davidi. Antarc Sci 26:15–22
Smith T, Wharton DA, Marshall CJ (2008) Cold tolerance of an Antarctic nematode that survives intracellular freezing: comparisons with other nematode species. J Comp Physiol B 178:93–100
Treonis AM, Wall DH, Virginia RA (2000) The use of anhydrobiosis by soil nematodes in the Antarctic Dry Valleys. Func Ecol 14:460–467
Wharton DA (1998) Comparison of the biology and freezing tolerance of Panagrolaimus davidi, an Antarctic nematode, from field samples and cultures. Nematologica 44:643–653
Wharton DA (2003) The environmental physiology of Antarctic terrestrial nematodes: a review. J Comp Physiol B 173:621–628
Wharton DA (2011) Cold tolerance. In: Perry RN, Wharton DA (eds) Molecular and physiological basis of nematode survival. CABI Publishing, Wallingford, pp 182–204
Wharton DA, Block W (1993) Freezing tolerance of some Antarctic nematodes. Func Ecol 7:578–584
Wharton DA, Brown IM (1989) A survey of terrestrial nematodes from the McMurdo Sound region, Antarctica. NZ J Zool 16:467–470
Wharton DA, Ferns DJ (1995) Survival of intracellular freezing by the Antarctic nematode Panagrolaimus davidi. J Exp Biol 198:1381–1387
Wharton DA, Rowland JJ (1984) A thermoelectric microscope stage for the measurement of the supercooling points of microscopic organisms. J Microsc 134:299–305
Wharton DA, Goodall G, Marshall CJ (2002) Freezing rate affects the survival of a short-term freezing stress in Panagrolaimus davidi, an Antarctic nematode that survives intracellular freezing. CryoLetters 23:5–10
Wharton DA, Goodall G, Marshall CJ (2003) Freezing survival and cryoprotective dehydration as cold tolerance mechanisms in the Antarctic nematode Panagrolaimus davidi. J Exp Biol 206:215–221
Wharton DA, Mutch JS, Wilson PW, Marshall CJ, Lim M (2004) A simple ice nucleation spectrometer. CryoLetters 25:335–340
Wharton DA, Barrett J, Goodall G, Marshall CJ, Ramløv H (2005a) Ice-active proteins from the Antarctic nematode Panagrolaimus davidi. Cryobiol 51:198–207
Wharton DA, Downes MF, Goodall G, Marshall CJ (2005b) Freezing and cryoprotective dehydration in an Antarctic nematode (Panagrolaimus davidi) visualised using a freeze substitution technique. Cryobiol 50:21–28
Acknowledgments
We would like to thank Antarctica New Zealand for the support of our Antarctic studies (event no. KO66), Karen Judge and Amy Armstrong for technical assistance and the financial support of the University of Otago (PBRF: PL.104033.01.S.FZ.75). MRR would like to acknowledge the support of a Kelly Tarlton’s Antarctica New Zealand Scholarship and the Fanny Evans Postgraduate Scholarship for Women. MRR would also like to thank Konstanze Gebauer for assistance in the field.
Conflict of interest
The authors declare no conflict of interest.
Ethical standard
This work was done in accordance with the procedures of the University of Otago’s Animal Ethics Committee.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by I. D. Hume.
Rights and permissions
About this article
Cite this article
Wharton, D.A., Raymond, M.R. Cold tolerance of the Antarctic nematodes Plectus murrayi and Scottnema lindsayae . J Comp Physiol B 185, 281–289 (2015). https://doi.org/10.1007/s00360-014-0884-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-014-0884-2