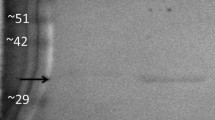
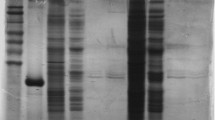
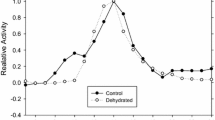
Abstract
The African clawed frog, Xenopus laevis, is able to withstand extremely arid conditions by estivating, in conjunction with dehydration tolerance and urea accumulation. Estivating X. laevis reduce their metabolic rate and recruit anaerobic glycolysis, driven by lactate dehydrogenase (LDH; E.C. 1.1.1.27) enzymes that reversibly convert pyruvate and NADH to lactate and NAD+, to meet newly established ATP demands. The present study investigated purified LDH from the liver of dehydrated and control X. laevis. LDH from dehydrated liver showed a significantly higher K m for l-lactate (1.74 fold), NAD+ (2.41 fold), and pyruvate (1.78 fold) in comparison to LDH from the liver of control frogs. In the presence of physiological levels of urea found in dehydrated animals, the K m values obtained for dehydrated LDH all returned to control LDH K m values. Dot blot analysis showed post-translational modifications may be responsible for the kinetic modification as the dehydrated form of LDH showed more phosphorylated serine residues (1.54 fold), less methylated lysine residues (0.43 fold), and a higher level of ubiquitination (1.90 fold) in comparison to control LDH. The physiological consequence of dehydration-induced LDH modification appears to adjust LDH function in conjunction with urea levels in dehydrated frogs. When urea levels are high during dehydration, LDH retains its normal function. Yet, as urea levels drop during rehydration, LDH function is reduced, possibly shunting pyruvate to the TCA cycle.





Similar content being viewed by others
Abbreviations
- ANOVA:
-
Analysis of variance
- ATP:
-
Adenosine triphosphate
- BWCi :
-
Initial body water content
- DEAE+ :
-
Diethylaminoethanol
- DSF:
-
Differential scanning fluorimetry
- EDTA:
-
Ethylene diamine tetraacetic acid
- EGTA:
-
Ethylene glycol tetraacetic acid
- IgG:
-
Immunoglobulin G
- K m :
-
Michaelis constant
- LDH:
-
Lactate dehydrogenase
- M d :
-
Measured mass
- M i :
-
Initial mass
- MW:
-
Molecular weight
- NAD+ :
-
Nicotinamide adenine dinucleotide
- NADH:
-
Reduced form of nicotinamide adenine dinucleotide
- PEG:
-
Polyethylene glycol
- PMSF:
-
Phenylmethanesulfonyl fluoride
- PVA:
-
Polyvinyl alcohol
- Rf:
-
Distance migrated over the gel length
- SDS-PAGE:
-
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis
- SEM:
-
Standard error of the mean
- TBS:
-
Tris-buffered saline
- TBST:
-
Tris-buffered saline with 0.05 % Tween-20
- TCA cycle:
-
Tricarboxylic acid cycle
- T m :
-
Melting point
- V max :
-
Maximum initial velocity of the enzyme catalyzed reaction
References
Abboud J, Storey KB (2013) Novel control of lactate dehydrogenase from the freeze tolerant wood frog: role of posttranslational modifications. PeerJ 1:e12. doi:10.7717/peerj.12
Atha DH, Ingham KC (1981) Mechanism of precipitation of proteins by polyethylene glycols. Analysis in terms of excluded volume. J Biol Chem 256(23):12108–12117
Balinsky JB, Cragg MM, Baldwin E (1961) The adaptation of amphibian waste nitrogen excretion to dehydration. Comp Biochem Phys 3:236–244
Balinsky JB, Choritz EL, Coe CG, van der Schans GS (1967) Amino acid metabolism and urea synthesis in naturally aestivating Xenopus laevis. Comp Biochem Phys 22(1):59–68
Bedford MT, Richard S (2005) Arginine methylation an emerging regulator of protein function. Mol Cell 18(3):263–272
Biggar KK, Dawson NJ, Storey KB (2012) Real-time protein unfolding: a method for determining the kinetics of native protein denaturation using a quantitative real-time thermocycler. Biotechniques 53(4):231–238
Brooks SP (1992) A simple computer program with statistical tests for the analysis of enzyme kinetics. Biotechniques 13(6):906–911
Burg MB, Peters EM, Bohren KM, Gabbay KH (1999) Factors affecting counteraction by methylamines of urea effects on aldose reductase. Proc Natl Acad Sci USA 96(11):6517–6522
Churchill TA, Storey KB (1994) Effects of dehydration on organ metabolism in the frog Pseudacris crucifer––hyperglycemic responses to dehydration mimic freezing-induced cryoprotectant production. J Comp Phys B 164(6):492–498
Clarke S (1993) Protein methylation. Curr Opin Cell Biol 5(6):977–983
Dawson NJ, Bell RA, Storey KB (2013) Purification and properties of white muscle lactate dehydrogenase from the anoxia-tolerant turtle, the red-eared slider, Trachemys scripta elegans. Enzyme Res 2013:784973
Fan J, Hitosugi T, Chung TW, Xie J, Ge Q, Gu TL, Polakiewicz RD, Chen GZ, Boggon TJ, Lonial S, Khuri FR, Kang S, Chen J (2011) Tyrosine phosphorylation of lactate dehydrogenase A is important for NADH/NAD(+) redox homeostasis in cancer cells. Mol Cell Biol 31(24):4938–4950
Franklin E, Mantle T, Dunne A (2013) Activation of human biliverdin-IX alpha reductase by urea: generation of kinetically distinct forms during the unfolding pathway. Biochim Biophys Acta 1834(12):2573–2578
Garza-Ramos G, Fernandez-Velasco DA, Ramirez L, Shoshani L, Darszon A, Tuena de Gomez-Puyou M, Gomez-Puyou A (1992) Enzyme activation by denaturants in organic solvent systems with a low water content. Eur J Biochem 205(2):509–517
Hermes-Lima M, Storey JM, Storey KB (1998) Antioxidant defenses and metabolic depression. The hypothesis of preparation for oxidative stress in land snails. Comp Biochem Physiol B 120(3):437–448
Hillman SS (1978a) Roles of oxygen delivery and electrolyte levels in dehydrational death of Xenopus laevis. J Comp Physiol 128(2):169–175
Hillman SS (1978b) Some effects of dehydration on internal distribution of water and solutes in Xenopus laevis. Comp Biochem Physiol A 61:303–307
Hillman SS, Withers PC, Drewes RC, Hillyard SD (2009) Ecological and environmental physiology of amphibians. Oxford University Press, Oxford
Huang J, Berger SL (2008) The emerging field of dynamic lysine methylation of non-histone proteins. Curr Opin Genet Dev 18(2):152–158
Janssens PA (1964) Urea production + transaminase activity in Xenopus laevis Daudin. Comp Biochem Phys 13(3):217
Joanisse DR, Storey KB (1996) Oxidative damage and antioxidants in Rana sylvatica the freeze-tolerant wood frog. Am J Physiol Reg I 271(3):R545–R553
Jorgensen CB (1997) Urea and amphibian water economy. Comp Biochem Physiol A 117(2):161–170
Malik AI, Storey KB (2009) Activation of extracellular signal-regulated kinases during dehydration in the African clawed frog, Xenopus laevis. J Exp Biol 212(Pt 16):2595–2603
Martin C, Zhang Y (2005) The diverse functions of histone lysine methylation. Nat Rev Mol Cell Biol 6(11):838–849
Merkle S (1989) Long-term starvation in Xenopus laevis Daudin-III. Effects on enzymes in several tissues. Comp Biochem Physiol B 94(4):783–788
Merkle S, Hanke W (1988) Long-term starvation in Xenopus laevis Daudin-II. Effects on several organs. Comp Biochem Physiol A 90(3):491–495
Niesen FH, Berglund H, Vedadi M (2007) The use of differential scanning fluorimetry to detect ligand interactions that promote protein stability. Nat Protoc 2(9):2212–2221
Onishi Y, Hirasaka K, Ishihara I, Oarada M, Goto J, Ogawa T, Suzue N, Nakano S, Furochi H, Ishidoh K, Kishi K, Nikawa T (2005) Identification of mono-ubiquitinated LDH-A in skeletal muscle cells exposed to oxidative stress. Biochem Biophys Res Commun 336(3):799–806
Ramnanan CJ, Allan ME, Groom AG, Storey KB (2009) Regulation of global protein translation and protein degradation in aerobic dormancy. Mol Cell Biochem 323(1–2):9–20
Romspert AP (1976) Osmoregulation of African clawed frog, Xenopus laevis, in hypersaline media. Comp Biochem Phys A 54(2):207–210
Storey KB, Storey JM (2004a) Metabolic rate depression in animals: transcriptional and translational controls. Biol Rev Cam Philos Soc 79(1):207–233
Storey KB, Storey JM (2004b) Oxygen limitation and metabolic rate depression. In: Functional metabolism: regulation and adaptation. Wiley, New York, pp 415–442
Storey KB, Storey JM (2004c) Physiology, biochemistry and molecular biology of vertebrate freeze tolerance: the wood frog. In: Benson E, Fuller B, Lane N (eds) Life in the frozen state. CRC Press, Boca Raton, pp 243–274
Tinsley RC, Kobel HR (1996) The biology of Xenopus. Oxford University Press, Oxford
Varadan R, Walker O, Pickart C, Fushman D (2002) Structural properties of polyubiquitin chains in solution. J Mol Biol 324(4):637–647
Woods AK, Storey KB (2005) Effects of hibernation on multicatalytic proteinase complex in thirteen-lined ground squirrels, Spermophilus tridecemlineatus. Mol Cell Biochem 271(1–2):205–213
Wray S, Wilkie DR (1995) The relationship between plasma urea levels and some muscle trimethylamine levels in Xenopus laevis––a P-31 and N-14 nuclear-magnetic-resonance study. J Exp Biol 198(2):373–378
Xiong ZJ, Storey KB (2012) Regulation of liver lactate dehydrogenase by reversible phosphorylation in response to anoxia in a freshwater turtle. Comp Biochem Phys B 163(2):221–228
Yancey PH, Somero GN (1978) Urea-requiring lactate-dehydrogenases of marine Elasmobranch fishes. J Comp Physiol 125(2):135–141
Yasykova MY, Petukhov SP, Muronetz VI (2000) Phosphorylation of lactate dehydrogenase by protein kinases. Biochemistry (Mosc) 65(10):1192–1196
Acknowledgments
The authors thank JM Storey for helpful discussions during the preparation of this manuscript. This research was supported by a discovery grant from the Natural Sciences and Engineering Research Council (NSERC) of Canada (6793 to KBS). BAK was supported by a NSERC Post Doctoral Fellowship and NJD was supported by an Ontario Graduate Scholarship.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by I. D. Hume.
B. A. Katzenback and N. J. Dawson contributed equally.
Rights and permissions
About this article
Cite this article
Katzenback, B.A., Dawson, N.J. & Storey, K.B. Purification and characterization of a urea sensitive lactate dehydrogenase from the liver of the African clawed frog, Xenopus laevis . J Comp Physiol B 184, 601–611 (2014). https://doi.org/10.1007/s00360-014-0824-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-014-0824-1