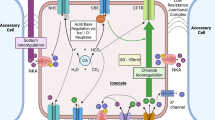
Abstract
In seawater-acclimated rainbow trout (Oncorhynchus mykiss), base secretion into the intestine is a key component of the intestinal water absorption that offsets osmotic water loss to the marine environment. Acid–base balance is maintained by the matched excretion of acid equivalents via other routes, presumably the gill and/or kidney. The goal of the present study was to examine acid–base balance in rainbow trout upon transfer to more dilute environments, conditions under which base excretion into the intestine is predicted to fall, requiring compensatory adjustments of acid excretion at the gill and/or kidney if acid–base balance is to be maintained. Net acid excretion via the gill/kidney and rectal fluid, and blood acid–base status were monitored in seawater-acclimated rainbow trout maintained in seawater or transferred to iso-osmotic conditions. As predicted, transfer to iso-osmotic conditions significantly reduced base excretion into the rectal fluid (by ~48%). Transfer to iso-osmotic conditions also significantly reduced the excretion of titratable acidity via extra-intestinal routes from 183.4 ± 71.3 to −217.5 ± 42.7 μmol kg−1 h−1 (N = 7). At the same time, however, ammonia excretion increased significantly during iso-osmotic transfer (by ~72%) so that the apparent overall reduction in net acid excretion (from 419.7 ± 92.9 to 189.2 ± 76.5 μmol kg−1 h−1; N = 7) was not significant. Trout maintained blood acid–base status during iso-osmotic transfer, although arterial pH was significantly higher in transferred fish than in those maintained in seawater. To explore the mechanisms underlying these adjustments of acid–base regulation, the relative mRNA expression and where possible, activity of a suite of proteins involved in acid–base balance were examined in intestine, gill and kidney. At the kidney, reduced mRNA expression of carbonic anhydrase (CA; cytosolic and membrane-associated CA IV), V-type H+-ATPase, and Na+/HCO3 − co-transporter were consistent with a reduced role in net acid excretion following iso-osmotic transfer. Changes in relative mRNA expression and/or activity at the intestine and gill were consistent with the roles of these organs in osmotic rather than acid–base regulation. Overall, the data emphasize the coordination of acid–base, osmoregulatory and ionoregulatory processes that occur with salinity transfer in a euryhaline fish.







Similar content being viewed by others
References
Ando M, Subramanyam MVV (1990) Bicarbonate transport systems in the intestine of the seawater eel. J Exp Biol 150:381–394
Bath RN, Eddy FB (1979) Ionic and respiratory regulation in rainbow trout during rapid transfer to seawater. J Comp Physiol 134:351–357
Beyenbach KW (2004) Kidneys sans glomeruli. Am J Physiol 286:F811–F827
Boutilier RG, Heming TA, Iwama GK (1984) Appendix: Physicochemical parameters for use in fish respiratory physiology. In: Hoar WS, Randall DJ (eds) Fish Physiology. Academic Press, Inc., London, pp 403–430
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utlizing the principle of protein-dye binding. Anal Biochem 72:248–254
Brown JA, Oliver JA, Henderson IW, Jackson BA (1980) Angiotensin and single nephron glomerular function in the trout Salmo gairdneri. Am J Physiol 239:R509–R514
Buxton TB, Crockett JK, Moore WL 3rd, Moore WL Jr, Rissing JP (1979) Protein precipitation by acetone for the analysis of polyethylene glycol in intestinal perfusion fluid. Gastroenterology 76:820–824
Bystriansky JS, Richards JG, Schulte PM, Ballantyne JS (2006) Reciprocal expression of gill Na+/K+-ATPase α-subunit isoforms α1a and α1b during seawater acclimation of three salmonid fishes that vary in their salinity tolerance. J Exp Biol 209:1848–1858
Claiborne JB, Edwards SL, Morrison-Shetlar AI (2002) Acid-base regulation in fishes: cellular and molecular mechanisms. J Exp Zool 293:302–319
Cliff WH, Beyenbach KW (1992) Secretory renal proximal tubules in seawater- and freshwater-adapted killifish. Am J Physiol 262:F116
Colin DA, Nonnotte G, LeRay C, Nonnotte L (1985) Na transport and enzyme activities in the intestine of the freshwater and sea-water adapted trout (Salmo gairdnerii R.). Comp Biochem Physiol 81A:695–698
Cooper CA, Whittamore JM, Wilson RW (2010) Ca2+-driven intestinal HCO3 − secretion and CaCO3 precipitation in the European flounder in vivo: influences on acid-base regulation and blood gas transport. Am J Physiol 298:R870–R876
Evans RM (1979) Onset and rate of drinking in rainbow trout (Salmo gairdneri) following transfer to dilute seawater. Can J Zool 57:1863–1865
Evans DH, Piermarini PM, Choe KP (2005) The multifunctional fish gill: dominant site of gas exchange, osmoregulation, acid-base regulation, and excretion of nitrogenous waste. Physiol Rev 85:97–177
Fuentes J, Bury NR, Carroll S, Eddy FB (1996a) Drinking in Atlantic salmon presmolts (Salmo salar L.) and juvenile rainbow trout (Oncorhynchus mykiss Walbaum) in response to cortisol and sea water challenge. Aquaculture 141:129–137
Fuentes J, Soengas JL, Buceta M, Otero J, Rey P, Rebolledo E (1996b) Kidney ATPase response in seawater-transferred rainbow trout (Oncorhynchus mykiss). Effect of salinity and fish size. J Physiol Biochem 52:231–238
Fuentes J, Soengas JL, Rey P, Rebolledo E (1997) Progressive transfer to seawater enhances intestinal and branchial Na+-K+-ATPase activity in non-anadromous rainbow trout. Aquac Int 5:217–227
Gaumet F, Boeuf G, Truchot J-P, Nonnotte G (1994) Effects of environmental water salinity on blood acid-base status in juvenile turbot (Scophthalmus maximus L.). Comp Biochem Physiol 109A:985–994
Genz J, Taylor JR, Grosell M (2008) Effects of salinity on intestinal bicarbonate secretion and compensatory regulation of acid-base balance in Opsanus beta. J Exp Biol 211:2327–2335
Georgalis T, Gilmour KM, Yorston J, Perry SF (2006) The roles of cytosolic and membrane bound carbonic anhydrase in the renal control of acid-base balance in rainbow trout Oncorhynchus mykiss. Am J Physiol 291:F407–F421
Grosell M (2006) Intestinal anion exchange in marine fish osmoregulation. J Exp Biol 209:2813–2827
Grosell M (2011). Intestinal anion exchange in marine teleosts is involved in osmoregulation and contributes to the oceanic inorganic carbon cycle. Acta Physiol (in press)
Grosell M, Genz J (2006) Ouabain-sensitive bicarbonate secretion and acid absorption by the marine teleost fish intestine play a role in osmoregulation. Am J Physiol 291:R1145–R1156
Grosell M, Taylor JR (2007) Intestinal anion exchange in teleost water balance. Comp Biochem Physiol A 148:14–22
Grosell M, Wood CM, Wilson RW, Bury NR, Hogstrand C, Rankin JC, Jensen FB (2005) Bicarbonate secretion plays a role in chloride and water absorption of the European flounder intestine. Am J Physiol 288:R936–R946
Grosell M, Gilmour KM, Perry SF (2007) Intestinal carbonic anhydrase, bicarbonate- and proton carriers play a role in the acclimation of rainbow trout to seawater. Am J Physiol 293:R2099–R2111
Grosell M, Genz J, Taylor JR, Perry SF, Gilmour KM (2009a) The involvement of H+-ATPase and carbonic anhydrase in intestinal HCO −3 secretion in seawater-acclimated rainbow trout. J Exp Biol 212:1940–1948
Grosell M, Mager EM, Willisams C, Taylor JR (2009b) High rates of HCO3 − secretion and Cl− absorption against adverse gradients in the marine teleost intestine: the involvement of an electrogenic anion exchanger and H+-pump metabolon? J Exp Biol 212:1684–1696
Henry RP (1991) Techniques for measuring carbonic anhydrase activity in vitro: the electrometric delta pH and pH stat assays. In: Dodgson SJ, Tashian RE, Gros G, Carter ND (eds) The carbonic anhydrases: cellular physiology and molecular genetics. Plenum, New York, pp 119–126
Hickman CP Jr, Trump BF (1969) The kidney. In: Hoar WS, Randall DJ (eds) Fish Physiology, vol. 1. Academic Press, New York, pp 91–239
Holmes RM (1961) Kidney function in migrating salmonids. Rep Challenger Soc Camb 3:23
Ivanis G, Braun M, Perry SF (2008a) Renal expression and localization of SLC9A3 sodium/hydrogen exchanger and its possible role in acid-base regulation in freshwater rainbow trout (Oncorhynchus mykiss). Am J Physiol 295:R971–R978
Ivanis G, Esbaugh A, Perry SF (2008b) Branchial expression and localization of SLC9A2 and SLC9A3 sodium/hydrogen exchangers and their possible role in acid-base regulation in freshwater rainbow trout (Oncorhynchus mykiss). J Exp Biol 211:2467–2477
Kurita Y, Nakada T, Kato A, Doi H, Mistry AC, Chang M-H, Romero MF, Hirose S (2008) Identification of intestinal bicarbonate transporters involved in formation of carbonate precipitates to stimulate water absorption in marine teleost fish. Am J Physiol 294:R1402–R1412
Lin H, Randall DJ (1993) H+-ATPase activity in crude homogenates of fish gill tissue: inhibitor sensitivity and environmental and hormonal regulation. J Exp Biol 180:163–174
Marshall WS (2002) Na+, Cl−, Ca2+ and Zn2+ transport by fish gills: retrospective review and prospective synthesis. J Exp Zool 293:264–283
Marshall WS, Grosell M (2006) Ion transport, osmoregulation, and acid-base balance. In: Evans DH, Claiborne JB (eds) The physiology of fishes, 3rd edn. CRC Press, Boca Raton, pp 177–230
McCormick SD (1993) Methods for nonlethal gill biopsy and measurement of Na+, K+-ATPase activity. Can J Fish Aquat Sci 50:656–658
McCormick SD (1995) Hormonal control of gill Na+, K+-ATPase and chloride cell function. In: Wood CM, Shuttleworth TJ (eds) Cellular and molecular approaches to fish ionic regulation. Academic Press, San Diego, pp 285–315
McCormick SD, Regish AM, Christensen AK (2009) Distinct freshwater and seawater isoforms of Na+/K+-ATPase in gill chloride cells of Atlantic salmon. J Exp Biol 212:3994–4001
McDonald DG, Wood CM (1981) Branchial and renal acid and ion fluxes in the rainbow trout, Salmo gairdneri, at low environmental pH. J Exp Biol 93:101–118
Nilsen TO, Ebbesson LOE, Madsen SS, McCormick SD, Andersson E, Björnsson BT, Prunet P, Stefansson SO (2007) Differential expression of gill Na+, K+-ATPase a- and ß-subunits, Na+, K+, 2Cl− cotransporter and CFTR anion channel in juvenile anadromous and landlocked Atlantic salmon Salmo salar. J Exp Biol 210:2885–2896
Patrick M, Pärt P, Marshall WS, Wood CM (1997) Characterization of ion and acid-base transport in the fresh water adapted mummichog (Fundulus heteroclitus). J Exp Zool 279:208–219
Pelis RM, Renfro JL (2004) Role of tubular secretion and carbonic anhdyrase in vertebrate renal sulfate excretion. Am J Physiol 287:R491–R501
Perrott MN, Grierson CE, Hazon N, Balment RJ (1992) Drinking behaviour in sea water and fresh water teleosts, the role of the renin-angiotensin system. Fish Physiol Biochem 10:161–168
Perry SF, Gilmour KM (2006) Acid-base balance and CO2 excretion in fish: Unanswered questions and emerging models. Respir Physiol Neurobiol 154:199–215
Perry SF, Beyers ML, Johnson DA (2000a) Cloning and molecular characterisation of the trout (Oncorhynchus mykiss) vacuolar H+-ATPase B subunit. J Exp Biol 203:459–470
Perry SF, Dumont C, Johnson DA (2000b) A molecular investigation of the role of the branchial vacuolar H+-ATPase in acid-base balance and ionic regulation in rainbow trout (Oncorhynchus mykiss). Ion transfer across fish gills. Proceedings of an International Fish Physiology Symposium held July 23–27, 2000:29–44
Perry SF, Furimsky M, Bayaa M, Georgalis T, Shahsavarani A, Nickerson JG, Moon TW (2003) Integrated responses of Na+/HCO3 − cotransporters and V-type H+-ATPases in the fish gill and kidney during respiratory acidosis. Biochim Biophys Acta 1618:175–184
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29:e45
Richards JG, Semple JW, Bystriansky JS, Schulte PM (2003) Na+/K+-ATPase α-isoform switching in gills of rainbow trout (Oncorhynchus mykiss) during salinity transfer. J Exp Biol 206:4475–4486
Scott GR, Schulte PM, Wood CM (2006) Plasticity of osmoregulatory function in the killifish intestine: drinking rates, salt and water transport, and gene expression after freshwater transfer. J Exp Biol 209:4040–4050
Shehadeh ZH, Gordon MS (1969) The role of the intestine in salinity adaptation of the rainbow trout, Salmo gairdneri. Comp Biochem Physiol 30:397–418
Smith HW (1930) The absorption and excretion of water and salts by marine teleosts. Am J Physiol 93:480–505
Soivio A, Nynolm K, Westman K (1975) A technique for repeated sampling of the blood of individual resting fish. J Exp Biol 62:207–217
Sullivan GV, Fryer JN, Perry SF (1995) Immunolocalization of proton pumps (H+-ATPase) in pavement cells of rainbow trout gill. J Exp Biol 198:2619–2629
Sullivan GV, Fryer JN, Perry SF (1996) Localization of mRNA for the proton pump (H+-ATPase) and Cl−/HCO3 − exchanger in the rainbow trout gill. Can J Zool 74:2095–2103
Taylor JR, Mager EM, Grosell M (2010) Basolateral NBCe1 plays a rate-limiting role in transepithelial intestinal HCO3 − secretion, contributing to marine fish osmoregulation. J Exp Biol 213:459–468
Verdouw H, van Echted CJA, Dekkers EMJ (1978) Ammonia determination based on indophenol formation with sodium salicylate. Water Res 12:399–402
Walsh PJ, Blackwelder P, Gill KA, Danulat E, Mommsen TP (1991) Carbonate deposits in marine fish intestines: a new source of biomineralization. Limnol Oceanogr 36:1227–1232
Wilson RW, Grosell M (2003) Intestinal bicarbonate secretion in marine teleost fish: source of bicarbonate, pH sensitivity, and consequences for whole animal acid-base and calcium homeostasis. Biochim Biophys Acta 1618:163–174
Wilson RW, Wright PM, Munger RS, Wood CM (1994) Ammonia excretion in freshwater rainbow trout (Oncorhynchus mykiss) and the importance of gill boundary layer acidification: lack of evidence for Na+/NH4 + exchange. J Exp Biol 191:37–58
Wilson RW, Gilmour KM, Henry RP, Wood CM (1996) Intestinal base excretion in the seawater-adapted rainbow trout: a role in acid-base balance? J Exp Biol 199:2331–2343
Wilson RW, Wilson JM, Grosell M (2002) Intestinal bicarbonate secretion by marine teleost fish: why and how? Biochim Biophys Acta 1566:182–193
Wood CM, Marshall WS (1994) Ion balance, acid-base regulation, and chloride cell function in the common killifish, Fundulus heteroclitus: a euryhaline estuarine teleost. Estuaries 17:34–52
Acknowledgments
This study was supported by Natural Sciences and Engineering Research Council (NSERC) of Canada Discovery and Research Tools and Instruments grants to KMG and SFP, and a National Science Foundation (NSF) grant (IAB 0743903) to MG. JRT and JG received Journal of Experimental Biology Travelling Fellowships, and AJE was supported by an NSERC Postdoctoral Fellowship. The help of Branka Vulesevic was greatly appreciated. We are grateful to Mr. Ray Volk and Mr. Steven Emmonds of the Robertson Creek Hatchery (Department of Fisheries and Oceans, Port Alberni, BC, Canada) for their help in supplying the trout used for this study. Mr. Randy Dolighan (British Columbia Ministry of Environment, Nanaimo, BC, Canada) is thanked for providing the brood stock from which the trout used in the study were derived. The enthusiasm and tireless support of Dr. Bruce Cameron (BMSC Research Director) were invaluable—thank you.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by H.V. Carey.
Rights and permissions
About this article
Cite this article
Gilmour, K.M., Perry, S.F., Esbaugh, A.J. et al. Compensatory regulation of acid–base balance during salinity transfer in rainbow trout (Oncorhynchus mykiss). J Comp Physiol B 182, 259–274 (2012). https://doi.org/10.1007/s00360-011-0617-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-011-0617-8