Abstract
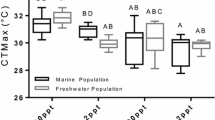
We characterized the degree of plasticity in thermal tolerance (assessed as critical thermal maxima; CTMax) and the relationship between thermal tolerance and underlying physiological and biochemical factors in two subspecies of a teleost fish, Fundulus heteroclitus. CTMax was not affected by repeated daily heat shock, but increased within a few days in response to warm acclimation. Loss of tolerance with acclimation to lowered temperatures occurred more slowly. Exposure to hypoxia decreased CTMax, and hyperoxia had no effect. CTMax showed a daily rhythm in both subspecies. Thermal acclimation changed the value of CTMax but did not affect the amplitude of the rhythm. Exposure to altered photoperiod had complex effects with a summer photoperiod producing a daily rhythm at higher CTMax than a spring photoperiod, and a winter photoperiod removing the rhythm. There was no daily rhythm in routine metabolic rate in either subspecies. There was no relationship between CTMax and the protein levels of the constitutive 70 and 90 kDa heat shock proteins (HSC70, HSP90β) in gill, or with mRNA levels of hsc70 in liver. There was a daily rhythm in the basal levels of the inducible hsp70-2 mRNA. Induction of hsp70-2 mRNA with mild heat shock occurred only in the evening and at night, and not during the day. These results demonstrate that there is substantial plasticity of thermal tolerance in killifish, and that this plasticity does not differ between subspecies. CTMax has a complex relationship with physiological and biochemical mechanisms that have been hypothesized to affect thermal tolerance.








Similar content being viewed by others
References
Alabaster JS, Welcomme RL (1962) Effect of concentration of dissolved oxygen on survival of trout and roach in lethal temperatures. Nature 194:107
Beitinger TL, Bennett WA, McCauley RW (2000) Temperature tolerances of North American freshwater fishes exposed to dynamic changes in temperature. Environ Biol Fishes 58:237–275
Bulger AJ (1984) A daily rhythm in heat tolerance in the salt marsh fish Fundulus heteroclitus. J Exp Zool 230:3571–3579
Bulger AJ, Tremaine SC (1985) Magnitude of seasonal effects on heat tolerance in Fundulus heteroclitus. Physiol Zool 58:197–204
Chomczynski P, Sacchi N (1987) Single-step method of RNA isolation by acid quanidinium thiocyanate–phenol–chloroform extraction. Anal Biochem 162:156–159
Fangue NA, Hofmeister M, Schulte PM (2006) Intraspecific variation in thermal tolerance and heat shock protein gene expression in common killifish, Fundulus heteroclitus. J Exp Biol 209:2859–2872
Fangue NA, Mandic M, Richard JG, Schulte PM (2008) Swimming performance and energetics as a function of temperature in killifish, Fundulus heteroclitus. Physiol Biochem Zool 81:389–401
Fangue NA, Richards JG, Schulte PM (2009) Do mitochondrial properties explain intraspecific variation in thermal tolerance? J Exp Biol 214:514–522
Fry FEJ (1971) The effect of environmental factors on the physiology of fish. In: Hoar WS, Randall DJ (eds) Fish physiol, vol 6. Academic Press, New York, pp 1–98
Garbuz DG, Zatsepina OG, Przhiboro AA, Yushenova I, Guzhova IV, Evgen’ev MB (2008) Larvae of related Diptera species from thermally contrasting habitats exhibit continuous up-regulation of heat shock proteins and high thermotolerance. Mol Ecol 17:4763–4777
Garland T, Huey RB, Bennett AF (1991) Phylogeny and coadaptation of thermal physiology in lizards: a reanalysis. Evolution 45:1969–1975
Healy TM, Tymchuk WE, Osborne EJ, Schulte PM (2010) The heat shock response of killifish (Fundulus heteroclitus): candidate gene and heterologous microarray approaches. Physiol Genomics 41:171–184
Hoffmann AA (2010) Physiological climatic limits in Drosophila: patterns and implications. J Exp Biol 213:870–880
Huey RB, Bennett AF (1990) Physiological adjustments to fluctuating thermal environments: an ecological and evolutionary perspective. In: Stress proteins in biology and medicine. Cold Spring Harbor Laboratory Press, Woodbury
Jones SJ, Mieszkowska N, Wethey DS (2009) Linking thermal tolerances and biogeography: Mytilus edulis (L.) at its southern limit on the east coast of the United States. Biol Bull 217:73–85
Klok CJ, Sinclair BJ, Chown SL (2004) Upper thermal tolerance and oxygen limitation in terrestrial arthropods. J Exp Biol 207:361–370
Nakano K, Iwama GK (2002) The 70-kDa heat shock protein response in two intertidal sculpins, Oligocottus maculosus and O. snyderi: relationship of hsp70 and thermal tolerance. Comp Biochem Physiol A Mol Integr Physiol 133:79–94
Pörtner HO (2002) Climate variations and the physiological basis of temperature dependent biogeography: systemic to molecular hierarchy of thermal tolerance in animals. Comp Biochem Physiol A Mol Integr Physiol 132:739–761
Portner HO, Farrell AP (2008) Physiology and climate change. Science 322:690–692
Powers DA, Dalessio PM, Lee E, DiMichele L (1986) The molecular ecology of Fundulus heteroclitus hemoglobin oxygen affinity. Am Zool 26:235–248
Powers DA, Smith M, Gonzalez-Villasenor I, DiMichele L, Crawford D, Bernardi G, Lauerman T (1993) A multidisciplinary approach to the selectionist/neutralist ccontroversy using the model teleost, F. heteroclitus. In: Futuyama D, Antonovics J (eds) Oxford survey of evolutionary biology. Oxford University Press, Oxford, pp 43–107
Rutledge CJ, Beitinger TL (1989) The effects of dissolved oxygen and aquatic surface respiration on the critical thermal maxima of three intermittent stream fishes. Environ Biol Fishes 24:137–143
Rutledge PS, Spotila JR, Easton DP (1987) Heat hardening in response to two types of heat shock in the lungless salamanders Eurycea bislineata and Desmognathus ochrophaeus. J Therm Biol 12:235–241
Schaefer J, Ryan A (2006) Developmental plasticity in the thermal tolerance of zebrafish Danio rerio. J Fish Biol 69:722–734
Sloman KA, Mandic M, Todgham AE, Fangue NA, Subrt P, Richards JG (2008) The response of the tidepool sculpin, Oligocottus maculosus, to hypoxia in laboratory, mesocosm and field environments. Comp Biochem Physiol A Mol Integr Physiol 149:284–292
Sorensen JG (2010) Application of heat shock protein expression for detecting natural adaptation and exposure to stress in natural populations. Curr Zool 56:703–713
Todgham AE, Hoaglund EA, Hofmann GE (2007) Is cold the new hot? Elevated ubiquitin-conjugated protein levels in tissues of Antarctic fish as evidence for cold-denaturation of proteins in vivo. J Comp Physiol B 177:857–866
Tomanek L (2008) The importance of physiological limits in determining biogeographical range shifts due to global climate change: the heat-shock response. Physiol Biochem Zool 81:709–717
Weatherley AH (1970) The effects of superabundant oxygen on thermal tolerance of goldfish. Biol Bull 139:229–238
Acknowledgments
The work presented here was supported financially through Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery and Discovery Accelerator grants to P.M.S. T.M.H. was supported by a NSERC Undergraduate Summer Research Award (USRA).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by H. V. Carey.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Healy, T.M., Schulte, P.M. Factors affecting plasticity in whole-organism thermal tolerance in common killifish (Fundulus heteroclitus). J Comp Physiol B 182, 49–62 (2012). https://doi.org/10.1007/s00360-011-0595-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-011-0595-x