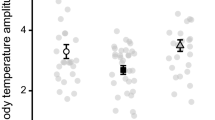
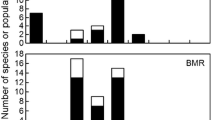
Abstract
Duration and start time of respirometry experiments have significant effects on the measurement of basal values for several commonly measured physiological variables (metabolic rate, evaporative water loss and body temperature). A longer measurement duration reduced values for all variables for all start times, and this was an effect of reduced animal activity rather than random sampling. However, there was also an effect of circadian rhythm on the timing of minimal physiological values. Experiment start time had a significant effect on time taken to reach minimal values for all variables, ranging from 0400 hours ± 38 min (body temperature, start time 2300 hours) to 0854 hours ± 52 min (evaporative water loss, start time 1700 hours). It also influenced the time of day that minimal values were obtained, ranging from 2224 hours ± 40 min (carbon dioxide production, start time 1500 hours) to 0600 hours ± 57 min (oxygen consumption, start time 2300 hours), and the minimum values measured. Consequently, both the measurement duration and the experiment start time should be considered in experimental design to account for both a handling and a circadian effect on the animal’s physiology. We suggest that experiments to measure standard physiological variables for small diurnal birds should commence between 1700 and 2100 hours, and measurement duration should be at least 9 h.





Similar content being viewed by others
Abbreviations
- α:
-
Active phase of the circadian cycle
- ρ:
-
Inactive phase of the circadian cycle
- BMR:
-
Basal metabolic rate
- D exp :
-
Time of day at the minimal value
- EWL:
-
Evaporative water loss
- S exp :
-
Experimental start time
- T b :
-
Body temperature
- T exp :
-
Time taken to reach the minimal value
- VO2:
-
Rate of oxygen consumption
- VCO2:
-
Rate of carbon dioxide production
References
Aschoff J, Pohl H (1970) Rhythmic variations in energy metabolism. Fed Proc 29:1541–1552
Benedict FG (1938) Vital energetics. A study in comparative basal metabolism. Carnegie Institute, Washington DC
Cooper CE, Withers PC (2009) Effects of measurement duration on the determination of basal metabolic rate and evaporative water loss of small marsupials; how long is long enough? Physiol Biochem Zool 82:438–446
Cooper CE, Withers PC (2010) The effect of sampling regime on the estimation of basal metabolic rate and standard evaporative water loss from flow-through respirometry. Physiol Biochem Zool 83:385–393
Cruz-Neto AP, Bozinovic F (2004) The relationship between diet quality and basal metabolic rate in endotherms: insights from intraspecific analysis. Physiol Biochem Zool 77:877–889
Elgar MA, Harvey PH (1987) Basal metabolic rates in mammals: allometry, phylogeny and ecology. Funct Ecol 1:25–36
Ellis HI, Gabrielsen GW (2001) Energetics of free-ranging seabirds. In: Schreiber EA, Burger J (eds) Biology of marine birds. CRC Press, Boca Raton, pp 359–408
Greenwald L, Stone WB, Cade TJ (1967) Physiological adjustments of the budgerygah (Melopsittacus undulatus) to dehydrating conditions. Comp Biochem Physiol 22:91–100
Hayes JP, Speakman JR, Racey PA (1992) Sampling bias in respirometry. Physiol Zool 65:604–619
Hill RW, Wyse GA, Anderson M (2004) Animal physiology. Sinauer Associates, Massachusetts
Hulbert AJ, Else PL (2004) Basal metabolic rate: history, composition, regulation, and usefulness. Physiol Biochem Zool 77:869–876
Koteja P (1991) On the relation between basal and field metabolic rates in birds and mammals. Funct Ecol 5:56–64
Krauchi K (2002) How is the circadian rhythm of core body temperature regulated? Clin Auton Res 12:147–149
Lovegrove BG (2003) The influence of climate on the basal metabolic rate of small mammals: a slow-fast continuum. J Comp Physiol B 173:87–112
McKechnie AE, Lovegrove BG (1999) Circadian metabolic responses to food deprivation in the black-shouldered kite. Condor 101:426–432
McNab BK (1966) An analysis of the body temperatures of birds. Condor 68:47–55
McNab BK (1988) Food habits and the basal rate of metabolism in birds. Oecologia 77:343–349
McNab BK (1997) On the utility of uniformity in the definition of basal rate of metabolism. Physiol Biochem Zool 70:718–720
McNab BK (2009) Ecological factors affect the level and scaling of avian BMR. Comp Biochem Physiol 152:22–45
Prinzinger R, Hanssler I (1980) Metabolism-weight relationship in some small nonpasserine birds. Cell Mol Life Sci 36:1299–1300
Rencher AC (1998) Multivariate statistical inference and applications. Wiley, New York
Rencher AC (2001) Methods of multivariate analysis, 2nd edn. Wiley, New York
Speakman JR, Krol E, Johnson MS (2004) The functional significance of individual variation in basal metabolic rate. Physiol Biochem Zool 77:900–915
Sturkie PD (1965) Avian physiology. Cornell University Press, New York
Weathers WW, Schoenbaechler DC (1976) Regulation of body temperature in the Budgerygah, Melopsittacus undulatus. Aust J Zool 24:39–47
Williams JB, Tieleman BI (2000) Flexibility in basal metabolic rate and evaporative water loss among hoopoe larks exposed to different environmental temperatures. J Exp Biol 203:3153–3159
Williams JB, Withers PC, Bradshaw SD, Nagy KA (1991) Metabolism and water flux of captive and free-living Australian parrots. Aust J Zool 39:131–142
Withers PC (1992) Comparative animal physiology. Saunders College Publishing, Philadelphia
Withers PC (2001) Design, calibration and calculation for flow-through respirometry systems. Aust J Zool 49:445–461
Withers PC, Cooper CE, Larcombe AN (2006) Environmental correlates of physiological variables in marsupials. Physiol Biochem Zool 79:453–473
Acknowledgments
We thank Professor Stephen Davies and Dr Beng Chua for assistance with the budgerigars, and Mr Charles Lacoste for help with maintenance of the equipment. This research was supported by funding from the University of Western Australia Research Grants Scheme (2008).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by I. D. Hume.
Rights and permissions
About this article
Cite this article
Page, A.J., Cooper, C.E. & Withers, P.C. Effects of experiment start time and duration on measurement of standard physiological variables. J Comp Physiol B 181, 657–665 (2011). https://doi.org/10.1007/s00360-011-0551-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-011-0551-9