Abstract
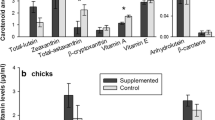
Carotenoids are well known for their immune-stimulant function in birds and other vertebrates. Moreover, they have potential antioxidant capacity, scavenging free radicals and protecting cell compartments from oxidation. Most essential carotenoids are fat soluble and could be stored for times of need especially in adipose tissues, built up by migratory birds as the main source of energy on long-distance flights. In an exclusive diet experiment, garden warblers (Sylvia borin) were fed ad libitum with an experimental diet, enriched with two different dose rates of carotenoids, or with control food, during the period of their first autumn migration. Plasma carotenoid content was measured via HPLC and chroma of plasma and fat examined with a spectrophotometer. Birds were infected with Isospora spp. and intensity of infection determined by oocyst counts 3 days post infection. Plasma lutein levels and chroma of subcutaneous fat stores were positively correlated and chroma values of these fat stores increased in the birds that got the higher dose rate, whereas they decreased significantly in the control group after infection with Isospora spp. Chroma of subcutaneous fat deposits in vivo and intensity of Isospora infection were negatively correlated. By measuring the chroma of fat deposits in vivo, we show that fat can be a reservoir for carotenoids. These colorful antioxidants are stored in the fat and taken from there in times of a higher demand, e.g. when mounting an immune response to parasites.




Similar content being viewed by others
References
Allen PC, Fetterer RH (2002) Interaction of dietary vitamin E with Eimeria maxima infections in chickens. Poult Sci 81:41–48
Bairlein F (1986) Ein standardisiertes Futter für Ernährungsuntersuchungen an omnivoren Kleinvögeln. J Ornithol 127:338–340
Bairlein F (1990) Nutrition and food selection in migratory birds. In: Gwinner E (ed) Bird migration. Springer, Berlin, pp 198–213
Bairlein F (1991) Sylvia borin (Boddaert 1783)—Gartengrasmücke. In: Glutz von Blotzheim UN, Bauer KM (eds) Handbuch der Vögel Mitteleur. Aula, Wiesbaden, pp 888–889
Bairlein F (1996) Fruit-eating in birds and its nutritional consequences. Comp Biochem Physiol 113:215–224
Bairlein F (2002) How to get fat: nutritional mechanisms of seasonal fat accumulation in migratory songbirds (rev). Naturwissenschaften 89:1–10
Bairlein F, Gwinner E (1994) Nutritional mechanisms and temporal control of migratory energy accumulation in birds. Annu Rev Nutr 14:187–215
Bairlein F, Totzke U (1992) New aspects on migratory physiology of trans-Saharan passerine migrants. Ornis Scand 23:244–250
Castaneda MP, Hirschler EM, Sams AR (2005) Skin pigmentation evaluation in broilers fed natural and synthetic pigments. Poult Sci 84:143–147
Chew BP (1993) Role of carotenoids in the immune response. J Dairy Sci 76:2804–2811
Constantini D, Møller AP (2008) Carotenoids are minor antioxidants for birds? Funct Ecol 22:367–370
Costantini D, Dell’Omo G (2006) Effects of T-cell-mediated immune response on avian oxidative stress. Comp Biochem Physiol 145:137–142
Costantini D, Cardinale M, Carere C (2007) Oxidative damage and anti-oxidant capacity in two migratory bird species at a stop-over site. Comp Biochem Physiol 144:363–371
Costantini D, Dell’Ariccia G, Lipp HP (2008) Long flights and age affect oxidative status of homing pigeons (Columba livia). J Exp Biol 211:377–381
Di Mascio P, Murphy ME, Sies H (1991) Antioxidant defense systems: the role of carotenoids, tocopherols, and thiols. Am J Clin Nutr 53:194–200
Dolnik OV (2002) Some aspects of the biology and host-parasite interactions of Isospora spp. (Protozoa: Coccidiida) of passerine birds. Dissertation, University of Oldenburg
Dolnik OV (2006) The relative stability of chronic Isospora sylvianthina (Protozoa: Apicomplexa) infection in blackcaps (Sylvia atricapilla): evaluation of a simplified method of estimating isosporan infection intensity in passerine birds. Parasitol Res 100:155–160
Fridolfsson AK, Ellegren H (1999) A simple and universal method for molecular sexing of non-ratite birds. J Avian Biol 30:116–121
Gray DA (1996) Carotenoids and sexual dichromatism in North American passerine birds. Am Nat 148:453–480
Gul M, Demircan B, Taysi S, Oztasan N, Gumustekin K, Siktar E, Polat F, Akar S, Akcay F, Dane S (2006) Effects of enduracne training and acute exhaustive exercise on antioxidant defense mechanisms in rat heart. Comp Biochem Physiol 143:239–245
Hamilton WD, Zuk M (1982) Heritable true fitness and bright birds: a role for parasites? Science 218:384–387
Hill GE (1991) Plumage coloration is a sexually selected indicator of male quality. Nature 350:337–339
Hill GE (1995) Interspecific variation in plasma hue in relation to carotenoid plumage pigmentation. Auk 112:1054–1057
Holden JM, Eldridge AL, Beecher GR, Buzzard IM, Bhagwat S, Davi CS, Douglass LW, Gebhardt S, Haytowitz D, Schakel S (1999) Carotenoid content of US foods: an update of the database. J Food Comp Anal 12:169–196
Horak P, Saks L, Karu U, Ots I, Surai P, McGraw K (2004) How coccidian parasites affect health and appearance of greenfinches. J Anim Ecol 71:935–947
Ji LL (1999) Antioxidants and oxidative stress in exercise. Proc Soc Exp Biol Med 222:283–292
Kaiser A (1993) A new multi-category classification of subcutaneous fat deposits of songbirds. J Field Ornithol 64:246–255
Kershaw E, Flier J (2004) Adipose tissue as an endocrine organ. J Clin Endocr Metabol 89:2548–2556
Kowaltowski AJ, Vercesi AE (1999) Mitochondrial damage induced by conditions of oxidative stress. Free Radic Biol Med 26:463–471
Krinsky NI (1993) Actions of carotinoids in biological systems. Ann Rev Nutr 13:561–587
Latscha T (1990) Carotenoids: their nature and significance in animal feeds. Roche, Basel (Publication 2175)
McGraw KJ (2006) Mechanics of carotenoid-based coloration. In: Hill GE, McGraw KJ (eds) Bird coloration mechanisms and measurements. Harvard University Press, Cambridge, pp 177–242
McGraw KJ, Toomey MB (2010) Carotenoid accumulation in the tissues of zebra finches: predictors of integumentary pigmentation and implications for carotenoid allocation strategies. Physiol Biochem Zool 83:97–109
McGraw KJ, Hill GE, Navara KJ, Parker RS (2004) Differential accumulation and pigmentation ability of dietary carotenoids in colorful finches. Physiol Biochem Zool 77:484–491
McGraw KJ, Crino OL, Medina-Jerez W, Nolan PM (2006) Effect of dietary carotenoid supplementation on food intake and immune function in a songbird with no carotenoid coloration. Ethology 112:1209–1216
McGraw KJ, Tourville E, Butler M (2008) A quantitative comparison of the commonly used methods for extracting carotenoids from avian plasma. Behav Ecol Sociobiol 62:1991–2002
McWilliams SR, Guglielmo C, Pierce B, Klaassen M (2004) Flying, fasting, and feeding in birds during migration: a nutritional and physiological ecology perspective. J Avian Biol 35:377–393
Møller AP, de Lope F, Saino N (2004) Parasitism, immunity, and arrival date in a migratory bird, the barn swallow. Ecology 85:206–219
Nachtigall W (1990) Wind tunnel measurements of long-time flights in relation to the energetics and water economy of migrating birds. In: Gwinner E (ed) Bird migration. Springer, Berlin, pp 319–327
Negro JJ, Figuerola J, Garrido J, Green AJ (2001) Fat stores in bird: an overlooked sink for carotenoid pigments? Funct Ecol 15:297–303
Olson VA, Owens IPF (1999) Costly sexual signals: are carotenoids rare, risky or required? Trends Ecol Evol 13:510–514
Ots I, Kerimov AB, Ivankina EV, Ilyina TA, Horak P (2001) Immune challenge affects basal metabolic activity in wintering great tits. Proc R Soc Lond B 268:1175–1181
Scott I, Evans PR (1992) The metabolic output of avian (Sturnus vulgaris, Calidris alpina) adipose tissue liver and skeletal muscle: implications for BMR/body mass relationships. Comp Biochem Physiol 103:329–332
Scott I, Mitchell PI, Evans PR (1996) How does variation body composition affect the basal metabolic rates of birds? Funct Ecol 10:307–313
Tella JL, Negro JJ, Rodríguez-Estrella R, Blanco G, Forero MG, Blázquez MC, Hiraldo F (1998) A comparison of spectrophotometry and color charts for evaluating total plasma carotenoids in wild birds. Physiol Zool 71:708–711
Wikelski M, Tarlow EM, Raim A, Diehl RH, Larkin RP, Visser H (2003) Costs of migration in free-flying songbirds. Nature 423:704
Acknowledgments
We are grateful to U. Strauß and A. Völk for help with bird maintenance. T. Klinner, U. Pianovska and J. Delingat helped with lab work. P. H. Becker and R. Nagel provided logistical support. O. Dolnik gave critical comments especially concerning parasitological aspects. The work benefited from remarks of two anonymous referees. All birds were taken from the wild and held under license of Ministry of Environment of Lower Saxony, Germany. All experiments were conducted under licence of Weser-Ems District Government, Lower Saxony, Germany (no. 509f-42502-32/12). The work was funded by the German Science Foundation (DFG).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by G. Heldmaier.
Rights and permissions
About this article
Cite this article
Metzger, B.J., Bairlein, F. Fat stores in a migratory bird: a reservoir of carotenoid pigments for times of need?. J Comp Physiol B 181, 269–275 (2011). https://doi.org/10.1007/s00360-010-0511-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-010-0511-9