Abstract
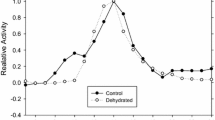
Glucose-6-phosphate dehydrogenase (G6PDH) and the pentose phosphate pathway play a key role in reductive biosynthesis and antioxidant defense, while diverting glucose from other cellular functions. G6PDH was isolated from liver of the wood frog, Rana sylvatica, a freeze tolerant species that uses glucose as a cryoprotectant. Analysis of kinetic parameters (K m and V max) of G6PDH showed a significant increase in K m G6P (from 98.2 ± 3.8 to 121 ± 5.3 μM) and K m NADP+ (from 65.5 ± 2.3 to 89.1 ± 4.8 μM) in frogs following freezing exposure, indicating lower affinity for G6PDH substrates in this state. Subsequent analyses indicated that differential phosphorylation of G6PDH between the two states was responsible for the altered kinetic properties. Thus, two differentially charged forms of G6PDH were resolved by DEAE ion-exchange chromatography and, compared with controls, the proportion of G6PDH activity in peak I decreased and in peak II increased in liver from frozen frogs. G6PDH in peak I had a K m G6P of 94.1 ± 1.1 μM and K m NADP+ of 61.2 ± 3.5 μM, whereas Peak II G6PDH showed higher values (K m G6P was 172 ± 4.3 μM, K m NADP+ was 98.2 ± 3.3 μM). G6PDH from each peak was incubated with ions and second messengers to stimulate the actions of protein kinases with results indicating that G6PDH can be phosphorylated by protein kinase G, protein kinase C, AMP-activated protein kinase, or calmodulin-dependent protein kinase. The data indicate that in control frogs, G6PDH is in a high phosphate form and displays a high substrate affinity, whereas in frozen frogs G6PDH is less phosphorylated, with lower substrate affinity.




Similar content being viewed by others
References
Bagnyukova TV, Storey KB, Lushchak VI (2003) Induction of oxidative stress in Rana ridibunda during recovery from winter hibernation. J Therm Biol 28:21–28
Bagnyukova TV, Chahrak OI, Lushchak VI (2006) Coordinated response of goldfish antioxidant defenses to environmental stress. Aquat Toxicol 78:325–331
Brooks SPJ (1992) A simple computer program for the analysis of enzyme kinetics. Biotechniques 13:906–911
Cappellini MD, Fiorelli G (2008) Glucose-6-phosphate dehydrogenase deficiency. Lancet 371:64–74
Carvalho SD, Negrão N, Bianco AC (1993) Hormonal regulation of malic enzyme and glucose-6-phosphate dehydrogenase in brown adipose tissue. Am J Physiol 264:E874–E881
Ciardiello MA, Camardella L, Carratore V, di Prisco G (1997) Enzymes in Antarctic fish: glucose-6-phosphate dehydrogenase and glutamate dehydrogenase. Comp Biochem Physiol 118A:1031–1036
Costa Rosa LF, Curi R, Murphy C, Newsholme P (1995) Effect of adrenaline and phorbol myristate acetate or bacterial lipopolysaccharide on stimulation of pathways of macrophage glucose, glutamine and O2 metabolism. Evidence for cyclic AMP-dependent protein kinase mediated inhibition of glucose-6-phosphate dehydrogenase and activation of NADP+-dependent malic enzyme. Biochem J 310:709–714
Costanzo JP, Lee RE (2005) Cryoprotection by urea in a terrestrially hibernating frog. J Exp Biol 208:4079–4089
Crerar M, David ES, Storey KB (1988) Electrophoretic analysis of liver glycogen phosphorylase activation in the freeze tolerant wood frog. Biochim Biophys Acta 971:72–84
Deane EE, Woo NYS (2005) Expression studies on glucose-6-phosphatase dehydrogenase in sea bream: effects of growth hormone, somatostatin, salinity and temperature. J Exp Biol 303A:676–688
Dieni CA, Storey KB (2008) Regulation of 5′-adenosine monophosphate deaminase in the freeze tolerant wood frog, Rana sylvatica. BMC Biochem 9:12
Dieni CA, Storey KB (2009) Creatine kinase regulation by reversible phosphorylation in frog muscle. Comp Biochem Physiol B 152:405–412
Greenway SC, Storey KB (2000) Activation of mitogen-activated protein kinases during natural freezing and thawing in the wood frog. Mol Cell Biochem 209:29–37
Gupte RS, Floyd BC, Kozicky M, George S, Ungvari ZI, Neito V, Wolin MS, Gupte SA (2009) Synergistic activation of glucose-6-phosphate dehydrogenase and NAD(P)H oxidase by Src kinase elevates superoxide in type 2 diabetic, Zucker fa/fa, rat liver. Free Radic Biol Med 47:219–228
Joanisse DR, Storey KB (1996) Oxidative damage and antioxidants in Rana sylvatica, the freeze tolerant wood frog. Am J Physiol 271:R545–R553
Kahn BB, Alquier T, Carling D, Hardie DG (2005) AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab 1:15–25
Keyse SM (2008) The regulation of stress-activated MAP kinase signalling by protein phosphatases. Topics Curr Genet 20:33–49
King PA, Rosholt MN, Storey KB (1993) Adaptations of plasma membrane glucose transport facilitate cryoprotectant distribution in freeze tolerant frogs. Am J Physiol 265:R1036–R1042
Kletzien RF, Harris PKW, Foellmi LA (1994) Glucose-6-phosphate dehydrogenase: a “housekeeping” enzyme subject to tissue-specific regulation by hormones, nutrients, and antioxidant stress. FASEB J 8:174–181
Kohan AB, Talukdar I, Walsh CM, Salati LM (2009) A role for AMPK in the inhibition of glucose-6-phosphate dehydrogenase by polyunsaturated fatty acids. Biochem Biophys Res Commun 388:117–121
MacDonald JA, Storey KB (1999) Protein phosphatase responses during freezing and thawing in wood frogs: control of liver cryoprotectant metabolism. Cryo Letters 20:297–306
Man AKY, Woo NYS (2008) Upregulation of metallothionein and glucose-6-phosphate dehydrogenase expression in silver sea bream, Sparus sarba, exposed to sublethal levels of cadmium. Aquat Toxicol 89:214–221
Pan S, World CJ, Kovacs CJ, Berk BC (2009) Glucose 6-phosphate dehydrogenase is regulated through c-Src-mediated tyrosine phosphorylation in endothelial cells. Arterioscler Thromb Vasc Biol 29:895–901
Park C, Marqusee S (2005) Pulse proteolysis: a simple method for quantitative determination of protein stability and ligand binding. Nat Methods 2:207–212
Pawson T, Scott JD (2005) Protein phosphorylation in signalling—50 years and counting. Trends Biochem Sci 30:286–290
Ramnanan CJ, Storey KB (2005) Glucose-6-phosphate dehydrogenase regulation during hypometabolism. Biochem Biophys Res Commun 339:7–16
Rider MH, Hussain N, Horman S, Dilworth SM, Storey KB (2006) Stress-induced activation of the AMP-activated protein kinase in the freeze tolerant frog Rana sylvatica. Cryobiology 53:297–309
Serpillon S, Floyd BC, Gupte RS, George S, Kozicky M, Neito V, Recchia F, Stanley W, Wollin MS, Gupte SA (2009) Superoxide production by NAD(P)H oxidase and mitochondria is increased in genetically obese and hyperglycemic rat heat and aorta before the development of cardiac dysfunction. The role of glucose-6-phosphate dehydrogenase-derived NADPH. Am J Physiol 297:H153–H162
Storey KB (1987a) Glycolysis and the regulation of cryoprotectant synthesis in liver of the freeze tolerant wood frog. J Comp Physiol B 157:373–380
Storey KB (1987b) Organ-specific metabolism during freezing and thawing in a freeze tolerant frog. Am J Physiol 253:R292–R297
Storey KB (2004) Strategies for exploration of freeze responsive gene expression: advances in vertebrate freeze tolerance. Cryobiology 48:134–145
Storey KB, Storey JM (1984) Biochemical adaptation for freezing tolerance in the wood frog, Rana sylvatica. J Comp Physiol B 155:29–36
Storey JM, Storey KB (1985) Triggering of cryoprotectant synthesis by the initiation of ice nucleation in the freeze tolerant frog, Rana sylvatica. J Comp Physiol B 156:191–195
Storey KB, Storey JM (1986) Freeze tolerant frogs: cryoprotectants and tissue metabolism during freeze-thaw cycles. Can J Zool 64:49–56
Storey KB, Storey JM (1988) Freeze tolerance in animals. Physiol Rev 68:27–84
Storey KB, Storey JM (2004) Metabolic rate depression in animals: transcriptional and translational controls. Biol Rev 79:207–233
Storey KB, Keefe D, Kourtz L, Storey JM (1991) Glucose-6-phosphate dehydrogenase in cold hardy insects: kinetic properties, freezing stabilization, and control of hexose monophosphate shunt activity. Insect Biochem 21:157–164
Tian W-N, Braunstein LD, Pang J, Stuhlmeier KM, Xi QC, Tian X, Stanton RC (1998) Importance of glucose-6-phosphate dehydrogenase activity for cell growth. J Biol Chem 273:10609–10617
Tian W-N, Braunstein LD, Apse K, Pang J, Rose M, Tian X, Stanton RC (1999) Importance of glucose-6-phosphate dehydrogenase activity in cell death. Am J Physiol 276:C1121–C1131
Vazquez Illanes MD, Storey KB (1993) 6-Phosphofructo-2-kinase and control of cryoprotectant synthesis in freeze tolerant frogs. Biochim Biophys Acta 1158:29–32
Wagle A, Jivraj S, Garlock GL, Stapleton SR (1998) Insulin regulation of glucose-6-phosphate dehydrogenase gene expression is rapamycin-sensitive and requires phosphatidylinositol 3-kinase. J Biol Chem 273:14968–14974
Wang X, Ma Y, Huang C, Wan Q, Li N, Bi Y (2008) Glucose-6-phosphate dehydrogenase plays a central role in modulating reduced glutathione levels in reed callus under salt stress. Planta 227:611–623
Xu Y, Osborne BW, Stanton RC (2005) Diabetes causes inhibition of glucose-6-phosphate dehydrogenase via activation of protein kinase A which contributes to oxidative stress in rat kidney cortex. Am J Physiol 289:F1040–F1047
Zhang Z, Apse K, Pang J, Stanton RC (2000) High glucose inhibits glucose-6-phosphate dehydrogenase via cAMP in aortic endothelial cells. J Biol Chem 275:40042–40047
Acknowledgments
We thank J.M. Storey for critical commentary on the manuscript. This work was supported by a discovery grant from the Natural Sciences and Engineering Research Council of Canada and the Canada Research Chairs program.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by H.V. Carey.
Rights and permissions
About this article
Cite this article
Dieni, C.A., Storey, K.B. Regulation of glucose-6-phosphate dehydrogenase by reversible phosphorylation in liver of a freeze tolerant frog. J Comp Physiol B 180, 1133–1142 (2010). https://doi.org/10.1007/s00360-010-0487-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-010-0487-5