Abstract
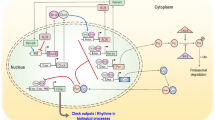
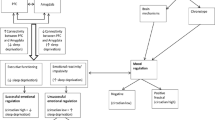
Daily variations in behaviour and physiology are controlled by a circadian timing system consisting of a network of oscillatory structures. In mammals, a master clock, located in the suprachiasmatic nuclei (SCN) of the hypothalamus, adjusts timing of other self-sustained oscillators in the brain and peripheral organs. Synchronisation to external cues is mainly achieved by ambient light, which resets the SCN clock. Other environmental factors, in particular food availability and time of feeding, also influence internal timing. Timed feeding can reset the phase of the peripheral oscillators whilst having almost no effect in shifting the phase of the SCN clockwork when animals are exposed (synchronised) to a light–dark cycle. Food deprivation and calorie restriction lead not only to loss of body mass (>15%) and increased motor activity, but also affect the timing of daily activity, nocturnal animals becoming partially diurnal (i.e. they are active during their usual sleep period). This change in behavioural timing is due in part to the fact that metabolic cues associated with calorie restriction affect the SCN clock and its synchronisation to light.




Similar content being viewed by others
References
Abe H, Kida M, Tsuji K, Mano T (1989) Feeding cycles entrain circadian rhythms of locomotor activity in CS mice but not in C57BL/6J mice. Physiol Behav 45:397–401
Andrade JP, Pereira PA, Silva SM, Sá SI, Lukoyanov NV (2004) Timed hypocaloric food restriction alters the synthesis and expression of vasopressin and vasoactive intestinal peptide in the suprachiasmatic nucleus. Brain Res 1022:226–233
Angeles-Castellanos M, Aguilar-Roblero R, Escobar C (2004) c-Fos expression in hypothalamic nuclei of food-entrained rats. Am J Physiol Regul Integr Comp Physiol 286:R158–R165
Armstrong S (1980) A chronometric approach to the study of feeding behavior. Neurosci Biobehav Rev 4:27–53
Asher G, Gatfield D, Stratmann M, Reinke H, Dibner C, Kreppel F, Mostoslavsky R, Alt FW, Schibler U (2008) SIRT1 regulates circadian clock gene expression through PER2 deacetylation. Cell 134:317–328
Barboza PS, Hume ID (2006) Physiology of intermittent feeding: integrating responses of vertebrates to nutritional deficit and excess. Physiol Biochem Zool 79:250–264
Bartness TJ, Wade GN (1984) Photoperiodic control of body weight and energy metabolism in Syrian hamsters (Mesocricetus auratus): role of pineal gland, melatonin, gonads, and diet. Endocrinology 114:492–498
Blank JL, Desjardins C (1985) Differential effects of food restriction on pituitary-testicular function in mice. Am J Physiol Regul Integr Comp Physiol 248:R181–R189
Blum ID, Patterson Z, Khazall R, Lamont EW, Sleeman MW, Horvath TL, Abizaid A (2009) Reduced anticipatory locomotor responses to scheduled meals in ghrelin receptor-deficient mice. Neuroscience 164:351–359
Boudard DL, Mendoza J, Hicks D (2009) Loss of photic entrainment at low illuminances in rats with acute photoreceptor degeneration. Eur J Neurosci 30:1527–1536
Bradbury MJ, Cascio CS, Scribner KA, Dallman MF (1991) Stress-induced adrenocorticotropin secretion: diurnal responses and decreases during stress in the evening are not dependent on corticosterone. Endocrinology 128:680–688
Caba M, González-Mariscal G (2009) The rabbit pup, a natural model of nursing anticipatory activity. Eur J Neurosci 30:1697–1706
Caldelas I, Feillet CA, Dardente H, Eclancher F, Malan A, Gourmelen S, Pévet P, Challet E (2005) Timed hypocaloric feeding and melatonin synchronize the suprachiasmatic clockwork in rats, but with opposite timing of behavioral output. Eur J Neurosci 22:921–929
Cambras T, Vilaplana J, Diez-Noguera A (1993) Effects of long-term restricted feeding on motor activity rhythm in the rat. Am J Physiol Regul Integr Comp Physiol 265:R467–R473
Canto C, Auwerx J (2009) Caloric restriction, SIRT1 and longevity. Trends Endocrinol Metab 20:325–331
Castillo MR, Hochstetler KJ, Travernier RJ, Greene DM, Bult-Ito A (2004) Entrainment of the master circadian clock by scheduled feeding. Am J Physiol Regul Integr Comp Physiol 287:R551–R555
Chacón F, Esquifino AI, Perello M, Cardinali DP, Spinedi E, Alvarez MP (2005) 24-Hour changes in ACTH, corticosterone, growth hormone, and leptin levels in young male rats subjected to calorie restriction. Chronobiol Int 22:253–265
Challet E (2007) Minireview: entrainment of the suprachiasmatic clockwork in diurnal and nocturnal mammals. Endocrinology 148:5648–5655
Challet E, Pévet P (2003) Interactions between photic and nonphotic stimuli to synchronize the master circadian clock in mammals. Front Biosci 8:S246–S257
Challet E, Malan A, Pévet P (1996a) Daily hypocaloric feeding entrains circadian rhythms of wheel-running and body temperature in rats kept in constant darkness. Neurosci Lett 211:1–4
Challet E, Pévet P, Malan A (1996b) Intergeniculate leaflet lesion and daily rhythms in food- restricted rats fed during daytime. Neurosci Lett 216:214–218
Challet E, Le Maho Y, Pévet P, Nobelis P, Malan A (1996c) Ventromedial hypothalamic lesions prevent the fasting-induced changes in day–night pattern of locomotor activity. Behav Brain Res 77:155–163
Challet E, Pévet P, Malan A (1997a) Effects of prolonged fasting and subsequent refeeding on free-running rhythms of temperature and locomotor activity in rats. Behav Brain Res 84:275–284
Challet E, Pévet P, Malan A (1997b) Lesion of the serotonergic terminals in the suprachiasmatic nuclei limits the phase advance of body temperature rhythm in food-restricted rats fed during daytime. J Biol Rhythms 12:235–244
Challet E, Pévet P, Lakhdar-Ghazal N, Malan A (1997c) Ventromedial nuclei of the hypothalamus are involved in the phase advance of temperature and activity rhythms in food-restricted rats fed during daytime. Brain Res Bull 43:209–218
Challet E, Pévet P, Vivien-Roels B, Malan A (1997d) Phase-advanced daily rhythms of melatonin, body temperature, locomotor activity in food-restricted rats fed during daytime. J Biol Rhythms 12:65–79
Challet E, Jacob N, Vuillez P, Pévet P, Malan A (1997e) Fos-like immunoreactivity in the circadian timing system of calorie-restricted rats fed at dawn: daily rhythms and light pulse-induced changes. Brain Res 770:228–236
Challet E, Bernard DJ, Turek FW (1998a) Lesions of glucose-responsive neurons impair synchronizing effects of calorie restriction in mice. Brain Res 801:244–250
Challet E, Solberg LC, Turek FW (1998b) Entrainment in calorie-restricted mice: conflicting zeitgebers and free-running conditions. Am J Physiol Regul Integr Comp Physiol 274:R1751–R1761
Challet E, Losee-Olson S, Turek FW (1999) Reduced glucose availability attenuates circadian responses to light in mice. Am J Physiol Regul Integr Comp Physiol 276:R1063–R1070
Challet E, Kolker DE, Turek FW (2000a) Metabolic influences on circadian rhythmicity in Siberian and Syrian hamsters exposed to long photoperiods. J Neuroendocrinol 12:69–78
Challet E, Takahashi JS, Turek FW (2000b) Nonphotic phase shifting in clock mutant mice. Brain Res 859:398–403
Challet E, Malan A, Turek FW, Van Reeth O (2004) Daily variations of blood glucose, acid–base state and PCO2 in rats: effect of light exposure. Neurosci Lett 355:131–135
Challet E, Mendoza J, Dardente H, Pévet P (2009) Neurogenetics of food anticipation. Eur J Neurosci 30:1676–1687
Cherel Y, El Omari B, Le Maho Y, Saboureau M (1995) Protein utilization during fasting with shallow and deep torpor in the European hedgehog (Erinaceus europaeus). J Comp Physiol B 164:653–658
Coleman GJ, Francis AJ (1991) Food deprivation and reinstatement phase shifts rat activity rhythms in constant light but not constant dark. Physiol Behav 50:167–171
Coleman GJ, O’Reilly HM, Armstrong SM (1989) Food-deprivation-induced phase shifts in Sminthopsis macroura froggatti. J Biol Rhythms 4:49–60
Cornish ER, Mrosovsky N (1965) Activity during food deprivation and satiation of six species of rodent. Anim Behav 13:242–248
Cuninkova L, Brown SA (2008) Peripheral circadian oscillators: interesting mechanisms and powerful tools. Ann N Y Acad Sci 1129:358–370
Daan S, Pittendrigh CS (1976) A functional analysis of circadian pacemakers in nocturnal rodents. II. The variability of phase–response curves. J Comp Physiol 106:253–266
Damiola F, Le Minh N, Preitner N, Kornmann B, Fleury-Olela F, Schibler U (2000) Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev 14:2950–2961
Dardente H, Cermakian N (2007) Molecular circadian rhythms in central and peripheral clocks in mammals. Chronobiol Int 24:195–213
Davidson AJ (2009) Lesion studies targeting food-anticipatory activity. Eur J Neurosci 30:1658–1664
Dkhissi-Benyahya O, Gronfier C, De Vanssay W, Flamant F, Cooper HM (2007) Modeling the role of mid-wavelength cones in circadian responses to light. Neuron 53:677–687
Duffy PH, Feuers R, Nakamura KD, Leakey J, Hart RW (1990) Effect of chronic caloric restriction on the synchronization of various physiological measures in old female Fischer 344 rats. Chronobiol Int 7:113–124
Feillet CA, Ripperger JA, Magnone MC, Dulloo A, Albrecht U, Challet E (2006) Lack of food anticipation in Per2 mutant mice. Curr Biol 16:2016–2022
Feillet CA, Mendoza J, Pévet P, Challet E (2008a) Restricted feeding restores rhythmicity in the pineal gland of arrhythmic suprachiasmatic-lesioned rats. Eur J Neurosci 28:2451–2458
Feillet CA, Mendoza J, Albrecht U, Pévet P, Challet E (2008b) Forebrain oscillators ticking with different clock hands. Mol Cell Neurosci 37:209–221
Froy O, Chapnik N, Miskin R (2008) The suprachiasmatic nuclei are involved in determining circadian rhythms during restricted feeding. Neuroscience 155:1152–1159
Giroud S, Blanc S, Aujard F, Bertrand F, Gilbert C, Perret M (2008) Chronic food shortage and seasonal modulations of daily torpor and locomotor activity in the grey mouse lemur (Microcebus murinus). Am J Physiol Regul Integr Comp Physiol 294:R1958–R1967
Giroud S, Perret M, Le Maho Y, Momken I, Gilbert C, Blanc S (2009) Gut hormones in relation to body mass and torpor pattern changes during food restriction and re-feeding in the gray mouse lemur. J Comp Physiol B 179:99–111
Gredilla R, Barja G (2005) Minireview: the role of oxidative stress in relation to caloric restriction and longevity. Endocrinology 146:3713–3717
Guilding C, Piggins HD (2007) Challenging the omnipotence of the suprachiasmatic timekeeper: are circadian oscillators present throughout the mammalian brain? Eur J Neurosci 25:3195–3216
Gutman R, Yosha D, Choshniak I, Kronfeld-Schor N (2007) Two strategies for coping with food shortage in desert golden spiny mice. Physiol Behav 90:95–102
Hakansson ML, Brown H, Ghilardi N, Skoda RC, Meister B (1998) Leptin receptor immunoreactivity in chemically defined target neurons of the hypothalamus. J Neurosci 18:559–572
Hall AC, Hoffmaster RM, Stern EL, Harrington ME, Bickar D (1997) Suprachiasmatic nucleus neurons are glucose sensitive. J Biol Rhythms 12:388–400
Hankins MW, Peirson SN, Foster RG (2008) Melanopsin: an exciting photopigment. Trends Neurosci 31:27–36
Hannibal J, Fahrenkrug J (2004) Target areas innervated by PACAP-immunoreactive retinal ganglion cells. Cell Tissue Res 316:99–113
Hara R, Wan K, Wakamatsu H, Aida R, Moriya T, Akiyama M, Shibata S (2001) Restricted feeding entrains liver clock without participation of the suprachiasmatic nucleus. Genes Cells 6:269–278
Harrington ME (1997) The ventral lateral geniculate nucleus and the intergeniculate leaflet: interrelated structures in the visual and circadian systems. Neurosci Biobehav Rev 21:705–727
Holmes MM, Mistlberger RE (2000) Food anticipatory activity and photic entrainment in food-restricted BALB/c mice. Physiol Behav 68:655–666
Honma KI, Von Goetz C, Aschoff J (1983) Effects of restricted daily feeding on freerunning circadian rhythms in rats. Physiol Behav 30:905–913
Honma KI, Honma S, Hiroshige T (1984) Feeding-associated corticosterone peak in rats under various feeding cycles. Am J Physiol Regul Integr Comp Physiol 246:R721–R726
Hoogerwerf WA, Hellmich HL, Cornelissen G, Halberg F, Shahinian VB, Bostwick J, Savidge TC, Cassone VM (2007) Clock gene expression in the murine gastrointestinal tract: endogenous rhythmicity and effects of a feeding regimen. Gastroenterology 133:1250–1260
Horvath TL (1998) An alternate pathway for visual signal integration into the hypothalamo-pituitary axis: retinorecipient intergeniculate neurons project to various regions of the hypothalamus and innervate neuroendocrine cells including those producing dopamine. J Neurosci 18:1546–1558
Hyun DH, Emerson SS, Jo DG, Mattson MP, de Cabo R (2006) Calorie restriction up-regulates the plasma membrane redox system in brain cells and suppresses oxidative stress during aging. Proc Natl Acad Sci USA 103:19908–19912
Inouye ST (1982a) Restricted daily feeding does not entrain circadian rhythms of the suprachiasmatic nucleus in the rat. Brain Res 232:194–199
Inouye ST (1982b) Ventromedial hypothalamic lesions eliminate anticipatory activities of restricted daily feeding schedules in the rat. Brain Res 250:183–187
Inouye ST (1983) Does the ventromedial hypothalamic nucleus contain a self-sustained circadian oscillator associated with periodic feedings? Brain Res 279:53–63
Ishida A, Mutoh T, Ueyama T, Bando H, Masubuchi S, Nakahara D, Tsujimoto G, Okamura H (2005) Light activates the adrenal gland: timing of gene expression and glucocorticoid release. Cell Metab 2:297–307
Johansson A, Fredriksson R, Winnergren S, Hulting AL, Schioth HB, Lindblom J (2008) The relative impact of chronic food restriction and acute food deprivation on plasma hormone levels and hypothalamic neuropeptide expression. Peptides 29:1588–1595
Kalsbeek A, Strubbe JH (1998) Circadian control of insulin secretion is independent of the temporal distribution of feeding. Physiol Behav 63:553–558
Kalsbeek A, Barassin S, van Heerikhuize JJ, van der Vliet J, Buijs RM (2000) Restricted daytime feeding attenuates reentrainment of the circadian melatonin rhythm after an 8-h phase advance of the light–dark cycle. J Biol Rhythms 15:57–66
Kilduff TS, Dube MG (1979) The effects of seasonal photoperiods on the activity of cotton rats and rice rats. J Mammal 60:169–176
King BM (2006) The rise, fall, and resurrection of the ventromedial hypothalamus in the regulation of feeding behavior and body weight. Physiol Behav 87:221–244
Kohsaka A, Laposky AD, Ramsey KM, Estrada C, Joshu C, Kobayashi Y, Turek FW, Bass J (2007) High-fat diet disrupts behavioral and molecular circadian rhythms in mice. Cell Metab 6:414–421
Koubi HE, Robin JP, Dewasmes G, Le Maho Y, Minaire Y (1991) Fasting-induced rise in locomotor activity in rats coincides with increased protein utilization. Physiol Behav 50:337–343
Le Sauter J, Hoque N, Weintraub M, Pfaff DW, Silver R (2009) Stomach ghrelin-secreting cells as food-entrainable circadian clocks. Proc Natl Acad Sci USA 106:13582–13587
Lovegrove BG, Raman J, Perrin MR (2001) Daily torpor in elephant shrews (Macroscelidea: Elephantulus spp.) in response to food deprivation. J Comp Physiol B 171:11–21
Lynn SE, Breuner CW, Wingfield JC (2003) Short-term fasting affects locomotor activity, corticosterone, and corticosterone-binding globulin in a migratory songbird. Horm Behav 43:150–157
Mahoney LB, Denny CA, Seyfried TN (2006) Caloric restriction in C57BL/6J mice mimics therapeutic fasting in humans. Lipids Health Dis 5:e13
Marchant EG, Mistlberger RE (1996) Entrainment and phase shifting of circadian rhythms in mice by forced treadmill running. Physiol Behav 60:657–663
Martínez-Merlos MT, Angeles-Castellanos M, Diaz-Munoz M, Aguilar-Roblero R, Mendoza J, Escobar C (2004) Dissociation between adipose tissue signals, behavior and the food-entrained oscillator. J Endocrinol 181:53–63
Masoro EJ (2005) Overview of caloric restriction and ageing. Mech Ageing Dev 126:913–922
Masoro EJ, Shimokawa I, Higami Y, McMahan CA, Yu BP (1995) Temporal pattern of food intake not a factor in the retardation of aging processes by dietary restriction. J Gerontol A Biol Sci Med Sci 50A:B48–B53
Masuda A, Oishi T (1995) Effects of restricted feeding on the light-induced body weight change and locomotor activity in the Djungarian hamster. Physiol Behav 58:153–159
Mendoza J, Challet E (2009) Brain clocks: from the suprachiasmatic nuclei to a cerebral network. Neuroscientist 15:477–488
Mendoza J, Angeles-Castellanos M, Escobar C (2005a) A daily palatable meal without food deprivation entrains the suprachiasmatic nucleus of rats. Eur J Neurosci 22:2855–2862
Mendoza J, Graff C, Dardente H, Pévet P, Challet E (2005b) Feeding cues alter clock gene oscillations and photic responses in the suprachiasmatic nuclei of mice exposed to a light–dark cycle. J Neurosci 25:1514–1522
Mendoza J, Pévet P, Challet E (2007) Circadian and photic regulation of clock and clock-controlled proteins in the suprachiasmatic nuclei of calorie-restricted mice. Eur J Neurosci 25:3691–3701
Mendoza J, Pévet P, Challet E (2008a) High-fat feeding alters the clock synchronization to light. J Physiol 586:5901–5910
Mendoza J, Drevet K, Pévet P, Challet E (2008b) Daily meal timing is not necessary for resetting the main circadian clock by calorie restriction. J Neuroendocrinol 20:251–260
Mistlberger RE (1991) Scheduled daily exercise or feeding alters the phase of photic entrainment in Syrian hamsters. Physiol Behav 50:1257–1260
Mistlberger RE (1993) Effects of scheduled food and water access on circadian rhythms of hamsters in constant light, dark, and light:dark. Physiol Behav 53:509–516
Mistlberger RE (1994) Circadian food-anticipatory activity: Formal models and physiological mechanisms. Neurosci Biobehav Rev 18:171–195
Mistlberger R (2009) Food-anticipatory circadian rhythms: concepts and methods. Eur J Neurosci 30:1718–1729
Mistlberger RE, Rechtschaffen A (1984) Recovery of anticipatory activity to restricted feeding in rats with ventromedial hypothalamic lesions. Physiol Behav 33:227–235
Mistlberger RE, Sinclair SV, Marchant EG, Neil L (1997) Phase shifts to refeeding in the Syrian hamster mediated by running activity. Physiol Behav 61:273–278
Mistlberger RE, Antle MC, Webb IC, Jones M, Weinberg J, Pollock MS (2003) Circadian clock resetting by arousal in Syrian hamsters: the role of stress and activity. Am J Physiol Regul Integr Comp Physiol 285:R917–R925
Mistlberger RE, Webb IC, Simon MM, Tse D, Su C (2006) Effects of food deprivation on locomotor activity, plasma glucose, and circadian clock resetting in Syrian hamsters. J Biol Rhythms 21:33–44
Moga MM, Moore RY (1996) Putative excitatory amino acid projections to the suprachiasmatic nucleus in the rat. Brain Res 743:171–177
Moga MM, Weis RP, Moore RY (1995) Efferent projections of the paraventricular thalamic nucleus in the rat. J Comp Neurol 359:221–238
Morgado E, Gordon MK, Miñana-Solis MC, Meza E, Levine S, Escobar C, Caba M (2008) Hormonal and metabolic rhythms associated with the daily scheduled nursing in rabbit pups. Am J Physiol Regul Integr Comp Physiol 295:R690–R695
Morin LP, Allen CN (2006) The circadian visual system, 2005. Brain Res Rev 51:1–60
Mrosovsky N (1996) Locomotor activity and non-photic influences on circadian clocks. Biol Rev 71:343–372
Nakahata Y, Sahar S, Astarita G, Kaluzova M, Sassone-Corsi P (2009) Circadian control of the NAD+ salvage pathway by CLOCK-SIRT1. Science 324:654–657
Nelson W (1988) Food restriction, circadian disorder and longevity of rats and mice. J Nutr 118:286–289
Oishi K, Sakamoto K, Ishida N (2003) Bimodal circadian expression of serotonin N-acetyltransferase mRNA in the retina of rats under restricted feeding. Neurosci Lett 351:21–24
Oishi K, Uchida D, Ohkura N, Doi R, Ishida N, Kadota K, Horie S (2009) Ketogenic diet disrupts the circadian clock and increases hypofibrinolytic risk by inducing expression of plasminogen activator inhibitor-1. Arterioscler Thromb Vasc Biol 29:1571–1577
Oomura Y (1983) Glucose as a regulator of neuronal activity. Adv Metab Disord 10:31–65
Prosser RA, Bergeron HE (2003) Leptin phase advances the rat suprachiasmatic circadian clock in vitro. Neurosci Lett 336:139–142
Ralph MR, Foster RG, Davis FC, Menaker M (1990) Transplanted suprachiasmatic nucleus determines circadian period. Science 247:975–978
Ramadori G, Lee CE, Bookout AL, Lee S, Williams KW, Anderson J, Elmquist JK, Coppari R (2008) Brain SIRT1: anatomical distribution and regulation by energy availability. J Neurosci 28:9989–9996
Ramsey KM, Yoshino J, Brace CS, Abrassart D, Kobayashi Y, Marcheva B, Hong HK, Chong JL, Buhr ED, Lee C, Takahashi JS, Imai S, Bass J (2009) Circadian clock feedback cycle through NAMPT-mediated NAD+ biosynthesis. Science 324:651–654
Redlin U (2001) Neural basis and biological function of masking by light in mammals: suppression of melatonin and locomotor activity. Chronobiol Int 18:737–758
Resuehr D, Olcese J (2005) Caloric restriction and melatonin substitution: effects on murine circadian parameters. Brain Res 1048:146–152
Ribeiro AC, Sawa E, Carren-Le Sauter I, Le Sauter J, Silver R, Pfaff DW (2007) Two forces for arousal: pitting hunger versus circadian influences and identifying neurons responsible for changes in behavioral arousal. Proc Natl Acad Sci USA 104:20078–20083
Robin JP, Boucontet L, Chillet P, Groscolas R (1998) Behavioral changes in fasting emperor penguins: evidence for a ‘refeeding signal’ linked to a metabolic shift. Am J Physiol Regul Integr Comp Physiol 274:R746–R753
Rowland N (1982) Failure by deprived hamsters to increase food intake: some behavioral and physiological determinants. J Comp Physiol Psychol 96:591–603
Rutter J, Reick M, Wu LC, McKnight SL (2001) Regulation of clock and NPAS2 DNA binding by the redox state of NAD cofactors. Science 293:510–514
Sakaguchi T, Takahashi M, Bray GA (1988) Diurnal changes in sympathetic activity: relation to food intake and to insulin injected into the ventromedial or suprachiasmatic nucleus. J Clin Invest 82:282–286
Scheer FA, Ter Horst GJ, van der Vliet J, Buijs RM (2001) Physiological and anatomic evidence for regulation of the heart by suprachiasmatic nucleus in rats. Am J Physiol Heart Circ Physiol 280:H1391–H1399
Shibata S, Liou SY, Ueki S, Oomura Y (1986) Inhibitory action of insulin on suprachiasmatic nucleus neurons in rat hypothalamic slice preparations. Physiol Behav 36:79–81
Silverman HJ, Zucker I (1976) Absence of post-fast food compensation in the golden hamster (Mesocricetus auratus). Physiol Behav 17:271–285
Smith RD, Turek FW, Takahashi JS (1992) Two families of phase–response curves characterize the resetting of the hamster circadian clock. Am J Physiol Regul Integr Comp Physiol 262:R1149–R1153
Steinlechner S, Heldmaier G, Becker H (1983) The seasonal cycle of body weight in the Djungarian hamster: photoperiodic control and the influence of starvation and melatonin. Oecologia (Berlin) 60:401–405
Stephan FK (2001) Food-entrainable oscillators in mammals. In: Takahashi JS, Turek FW, Moore RY (eds) Circadian clocks. Handbook of behavioral neurobiology, vol 12. Kluwer, New York, pp 223–246
Stokkan KA, Yamazaki S, Tei H, Sakaki Y, Menaker M (2001) Entrainment of the circadian clock in the liver by feeding. Science 291:490–493
Sutton GM, Perez-Tilve D, Nogueiras R, Fang J, Kim JK, Cone RD, Gimble JM, Tschop MH, Butler AA (2008) The melanocortin-3 receptor is required for entrainment to meal intake. J Neurosci 28:12946–12955
Szentirmai E, Kapás L, Sun Y, Smith RG, Krueger JM (2010) Restricted feeding-induced sleep, activity and body temperature changes in normal and preproghrelin deficient mice. Am J Physiol Regul Integr Comp Physiol 298:R467–R477
Takahashi JS, Turek FW, Moore RY (2001) Circadian clocks. Handbook of behavioral neurobiology, vol 12. Kluwer, New York
Thompson RH, Swanson LW (1998) Organization of inputs to the dorsomedial nucleus of the hypothalamus: a reexamination with fluorogold and PHAL in the rat. Brain Res Rev 27:89–118
Tosini G, Fukuhara C (2002) The mammalian retina as a clock. Cell Tissue Res 309:119–126
Unger J, McNeill TH, Moxley RT, White M, Moss A, Livingston JN (1989) Distribution of insulin receptor-like immunoreactivity in the rat forebrain. Neuroscience 31:143–157
Verwey M, Amir S (2009) Food-entrainable circadian oscillators in the brain. Eur J Neurosci 30:1650–1657
Vrang N, Mrosovsky N, Mikkelsen JD (2003) Afferent projections to the hamster intergeniculate leaflet demonstrated by retrograde and anterograde tracing. Brain Res Bull 59:267–288
Waddington Lamont E, Harbour VL, Barry-Shaw J, Renteria Diaz L, Robinson B, Stewart J, Amir S (2007) Restricted access to food, but not sucrose, saccharine, or salt, synchronizes the expression of Period2 protein in the limbic forebrain. Neuroscience 144:402–411
Wakamatsu H, Yoshinobu Y, Aida R, Moriya T, Akiyama M, Shibata S (2001) Restricted-feeding-induced anticipatory activity rhythm is associated with a phase shift of the expression of mPer1 and mPer2 mRNA in the cerebral cortex and hippocampus but not in the suprachiasmatic nucleus of mice. Eur J Neurosci 13:1190–1196
Weindruch R (1992) Effect of caloric restriction on age-associated cancers. Exp Gerontol 27:575–581
Wise DD, Shear JB (2004) Circadian tracking of nicotinamide cofactor levels in an immortalized suprachiasmatic nucleus cell line. Neuroscience 128:263–268
Wolf G (2006) Calorie restriction increases life span: a molecular mechanism. Nutr Rev 64:89–92
Yang XJ, Kow LM, Funabashi T, Mobbs CV (1999) Hypothalamic glucose sensor: similarities to and differences from pancreatic beta-cell mechanisms. Diabetes 48:1763–1772
Yannielli PC, Molyneux PC, Harrington ME, Golombek DA (2007) Ghrelin effects on the circadian system of mice. J Neurosci 27:2890–2895
Yi CX, van der Vliet J, Dai J, Yin G, Ru L, Buijs RM (2006) Ventromedial arcuate nucleus communicates peripheral metabolic information to the suprachiasmatic nucleus. Endocrinology 147:283–294
Zigman JM, Jones JE, Lee CE, Saper CB, Elmquist JK (2006) Expression of ghrelin receptor mRNA in the rat and the mouse brain. J Comp Neurol 494:528–548
Zvonic S, Ptitsyn AA, Conrad SA, Scott LK, Floyd ZE, Kilroy G, Wu X, Goh BC, Mynatt RL, Gimble JM (2006) Characterization of peripheral circadian clocks in adipose tissues. Diabetes 55:962–970
Acknowledgments
I wish to thank deeply Pr. André Malan and Pr. Paul Pévet for guidance and support as well as Dr. Ivette Caldelas, Dr. Céline Feillet, Dr. Caroline Graff-Trecherel and Dr. Jorge Mendoza for their critical help in gathering part of the work presented here. I am also grateful to Dr. Sylvie Massemin and Jeffrey Hubbard for their helpful comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by I. D. Hume.
Rights and permissions
About this article
Cite this article
Challet, E. Interactions between light, mealtime and calorie restriction to control daily timing in mammals. J Comp Physiol B 180, 631–644 (2010). https://doi.org/10.1007/s00360-010-0451-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-010-0451-4