Abstract
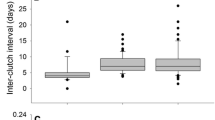
An experimental reduction of offspring number has been reported to result in enlargement of offspring size in lizards. We applied the “follicle excision” technique to a lacertid lizard (Takydromus septentrionalis) to examine whether this effect is generalisable to lizards. Of the 82 females that produced 3 successive clutches in the laboratory, 23 females underwent follicle excision after they oviposited the first clutch. Follicle excision reduced clutch size, but did not alter egg size. This result indicates that egg size is not altered during vitellogenesis in T. septentrionalis. Females undergoing follicle excision produced a third clutch (a second post-surgical clutch) as normally as did control females. Females switched from producing more but smaller eggs early in the breeding season to fewer but larger eggs later in the season. Our results indicate that female T. septentrionalis maximize reproductive success by diverting an optimal, rather than a higher, fraction of the available energy to individual offspring. This optimized allocation of the available energy to offspring production explains why follicle excision does not result in enlargement of egg size in this species. Our study provides evidence that an experimental reduction of offspring number does not always result in enlargement of offspring size in lizards.

Similar content being viewed by others
References
Ballinger RE (1983) Life-history variations. In: Huey RB, Pianka ER, Tinkle TW (eds) Lizard ecology: studies of a model organism. Harvard University Press, Cambridge, pp 241–260
Caley MJ, Schwarzkopf L, Shine R (2001) Does total reproductive effort evolve independently of offspring size? Evolution 55:1245–1248
Doughty P, Shine R (1997) Detecting life history trade-offs: measuring energy stores in “capital” breeders reveals costs of reproduction. Oecologia 110:508–513
Du WG, Lu YW, Ji X (2003) Utilization of lipids in northern grass lizards (Takydromus septentrionalis, Lacertidae) in Hangzhou in the breeding season. Zool Res 24:392–394
Du WG, Ji X, Shine R (2005a) Does body volume constrain reproductive output in lizards? Biol Lett 1:98–100
Du WG, Ji X, Zhang YP, Xu XF, Shine R (2005b) Identifying sources of variation in reproductive and life-history traits among five populations of a Chinese lizard (Takydromus septentrionalis, Lacertidae). Biol J Linn Soc 85:443–453
Ferguson GW, Snell HL (1986) Endogenous control of seasonal change of egg, hatchling, and clutch size of the lizard Sceloporus undulatus garmani. Herpetologica 42:185–191
James CD, Whitford WG (1994) An experimental study of phenotypic plasticity in the clutch size of a lizard. Oikos 70:44–48
Ji X, Braña F (2000) Among clutch variation in reproductive output and egg size in the wall lizard (Podarcis muralis) from a low land population of northern Spain. J Herpetol 34:54–60
Ji X, Zhou WH, Zhang XD, Gu HQ (1998) Sexual dimorphism and reproduction in the grass lizard, Takydromus septentrionalis. Russ J Herpetol 5:44–48
Lin ZH, Ji X (1998) The effects of thermal and hydric environments on incubating eggs and hatchlings of the grass lizard, Takydromus septentrionalis. Zool Res 19:439–445
Olsson M, Wapstra E, Olofsson C (2002) Offspring size-number strategies: experimental manipulation of offspring size in a viviparous lizard (Lacerta vivipara). Func Ecol 16:135–140
Reznick DN, Bryga H (1987) Life-history evolution guppies (Poecilia reticulata). I. Phenotypic and genetic changes in an induced experiment. Evolution 41:1370–1385
Reznick DN, Bryga H, Endler JA (1990) Experimentally induced life-history evolution in a natural population. Nature 346:357–359
Roff DA (1992) The evolution of life histories: theory and analysis. Chapman and Hall, New York
Sargent RC, Taylor PD, Gross MR (1987) Parental care and the evolution of egg size in fishes. Am Nat 129:32–46
Schwarzkopf L (1994) Measuring trade-offs: a review of studies of costs of reproduction in lizard. In: Vitt LJ, Pianka ER (eds) Lizard ecology: historical and experimental perspectives. Princeton University Press, Princeton, pp 7–29
Sinervo B (1998) Adaptation of maternal in the wide: path analysis of natural variation and experimental tests of causation. In: Mousseau TA, Fox CW (eds) Maternal effects as adaptations. Oxford University Press, Oxford, pp 288–306
Sinervo B, DeNardo DF (1996) Costs of reproduction in the wide: path analysis of natural variation and experimental tests of causation. Evolution 50:1299–1313
Sinervo B, Doughty P (1996) Interactive effects and timing or reproduction on offspring reproduction: experimental, maternal and quantitative genetics aspects. Evolution 50:1314–1327
Sinervo B, Licht P (1991a) Hormonal and physiological control of clutch size, egg size and egg shape in side-blotched lizards (Uta stansburiana): constraints on the evolution of lizard life histories. J Exp Zool 257:252–264
Sinervo B, Licht P (1991b) Proximate constraints on the evolution of egg size, number, and total clutch mass in lizards. Sciences 252:1300–1302
Smith CC, Fretwell SD (1974) The optimal balance between size and number of offspring. Am Nat 108:499–506
Stearns SC (1992) The evolutionary of life histories. Oxford University Press, Oxford
Winkler DW, Wallin K (1987) Offspring size and number: a life history model linking effort per offspring and total effort. Am Nat 129:708–720
Xu XF, Wu YL, Ou YY (2002) Water and energy content variation of the major energy reserves in adult grass lizards, Takydromus septentrionalis. Zool Res 23:44–48
Zhang YP, Ji X (2000) Ontogenetic changes of sexual dimorphism in head size and food habit in grass lizard, Takydromus septentrionalis. Zool Res 21:181–186
Acknowledgements
The Zhejiang Provincial Bureau of Forestry provided an official permit to collect lizards from the field. We thank Zhi-Hua Lin and Yu-Rong Jiang for their assistance both in the field and in the laboratory. We also thank Barry Sinervo for his very helpful comments. This work was funded by Nanjing Normal University and Zhejiang Provincial Natural Science Foundation (Research Project Grant RC97019).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by H.V. Carey
Rights and permissions
About this article
Cite this article
Ji, X., Diong, CH. Does follicle excision always result in enlargement of offspring size in lizards?. J Comp Physiol B 176, 521–525 (2006). https://doi.org/10.1007/s00360-006-0074-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-006-0074-y