Abstract
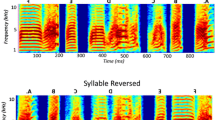
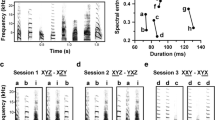
Songbirds are a well-established animal model to study the neural basis of learning, perception and production of complex vocalizations. In this system, telencephalic neurons in HVC present a state-dependent, highly selective response to auditory presentations of the bird’s own song (BOS). This property provides an opportunity to study the neural code behind a complex motor behavior. In this work, we explore whether changes in the temporal structure of the sound envelope can drive changes in the neural responses of highly selective HVC units. We generated an envelope-modified BOS (MOD) by reversing each syllable’s envelope but leaving the overall temporal structure of syllable spectra unchanged, which resulted in a subtle modification for each song syllable. We conducted in vivo electrophysiological recordings of HVC neurons in anaesthetized zebra finches (Taeniopygia guttata). Units analyzed presented a high BOS selectivity and lower response to MOD, but preserved the profile response shape. These results show that the temporal evolution of the sound envelope is being sensed by the avian song system and suggest that the biomechanical properties of the vocal apparatus could play a role in enhancing subtle sound differences.



Similar content being viewed by others
Abbreviations
- BOS:
-
Bird’s own song
- MOD:
-
Envelope-modified bird’s own song
- REV:
-
Reverse song
- CON:
-
Conspecific song
- PSTH:
-
Post-stimulus Time Histogram
- MAP:
-
Motif Activity Profile
- MRA:
-
Mean Relative Amplitude
- RRS:
-
Relative Response Strength
- RRV:
-
Relative Response Variability
- SC:
-
Spectral Centroid
- IRR:
-
Spectral Irregularity
- EMG:
-
Electromyography
References
Alonso R, Goller F, Mindlin GB (2014) Motor control of sound frequency in birdsong involves the interaction between air sac pressure and labial tension. Phys Rev E 89(3):032706
Amador A, Margoliash D (2013) A mechanism for frequency modulation in songbirds shared with humans. J Neurosci 33(27):11136–11144. https://doi.org/10.1523/JNEUROSCI.5906-12.2013
Amador A, Sanz Perl Y, Mindlin GB, Margoliash D (2013) Elemental gesture dynamics are encoded by song premotor cortical neurons. Nature 495:59–64. https://doi.org/10.1038/nature11967
Boari S, Perl YS, Amador A, Margoliash D, Mindlin GB (2015) Automatic reconstruction of physiological gestures used in a model of birdsong production. J Neurophysiol 114(5):2912–2922
Boersma P, Van Heuven V (2001) Speak and unSpeak with PRAAT. Glot International 5(9–10):341–347
Brainard MS, Doupe AJ (2000) Interruption of a basal ganglia–forebrain circuit prevents plasticity of learned vocalizations. Nature 404(6779):762–766
Bregman MR, Patel AD, Gentner TQ (2016) Songbirds use spectral shape, not pitch, for sound pattern recognition. Proc Natl Acad Sci USA 113(6):1666–1671
Caclin A, McAdams S, Smith BK, Winsberg S (2005) Acoustic correlates of timbre space dimensions: a confirmatory study using synthetic tones. J Acoust Soc Am 118(1):471–482
Dave AS, Margoliash D (2000) Song replay during sleep and computational rules for sensorimotor vocal learning. Science 290(5492):812–816
Dooling RJ, Prior NH (2017) Do we hear what birds hear in birdsong? Anim Behav 124:283–289
Dooling RJ, Lohr B, Dent ML (2000) Hearing in birds and reptiles. In: Dooling RJ, Fay RR (eds) Comparative hearing: birds and reptiles. Springer-Verlag, New York, pp 308–359
Dooling RJ, Leek MR, Gleich O, Dent ML (2002) Auditory temporal resolution in birds: discrimination of harmonic complexes. J Acoust Soc Am 112(2):748–759
Doupe AJ, Konishi M (1991) Song-selective auditory circuits in the vocal control system of the zebra finch. Proc Natl Acad Sci USA 88(24):11339–11343
Doupe AJ, Kuhl PK (1999) Birdsong and human speech: common themes and mechanisms. Annu Rev Neurosci 22:567–631
Drennan WR, Rubinstein JT (2008) Music perception in cochlear implant users and its relationship with psychophysical capabilities. J Rehabil Res Dev 45(5):779
Feher O, Wang HB, Saar S, Mitra PP, Tchernichovski O (2009) De novo establishment of wild-type song culture in the zebra finch. Nature 459(7246):564-U594. https://doi.org/10.1038/nature07994
Franz M, Goller F (2002) Respiratory units of motor production and song imitation in the zebra finch. Dev Neurobiol 51(2):129–141
Grace JA, Amin N, Singh NC, Theunissen FE (2003) Selectivity for conspecific song in the zebra finch auditory forebrain. J Neurophysiol 89(1):472–487
Iverson P, Krumhansl CL (1993) Isolating the dynamic attributes of musical timbre. J Acoust Soc Am 94(5):2595–2603
Kong Y-Y, Mullangi A, Marozeau J, Epstein M (2011) Temporal and spectral cues for musical timbre perception in electric hearing. J Speech Lang Hear Res 54(3):981–994
Krimphoff J, McAdams S, Winsberg S (1994) Caractérisation du timbre des sons complexes. II. Analyses acoustiques et quantification psychophysique. Le Journal de Physique IV 4(C5):C5–C625-C625-628
Lewicki MS (1996) Intracellular characterization of song-specific neurons in the zebra finch auditory forebrain. J Neurosci 16(18):5854–5863
Margoliash D (1983) Acoustic parameters underlying the responses of song-specific neurons in the white-crowned sparrow. J Neurosci 3(5):1039–1057
Margoliash D (1986) Preference for autogenous song by auditory neurons in a song system nucleus of the white-crowned sparrow. J Neurosci 6(6):1643–1661
Margoliash D, Fortune ES (1992) Temporal and harmonic combination-sensitive neurons in the zebra finch’s HVc. J Neurosci 12(11):4309–4326
Margoliash D, Fortune ES, Sutter ML, Yu AC, Wrenhardin BD, Dave A (1994) Distributed representation in the song system of Oscines: evolutionary implications and functional consequences. Brain Behav Evol 44(4–5):247–264
McAdams S, Winsberg S, Donnadieu S, De Soete G, Krimphoff J (1995) Perceptual scaling of synthesized musical timbres: common dimensions, specificities, and latent subject classes. Psychol Res 58(3):177–192
Mindlin GB, Gardner TJ, Goller F, Suthers R (2003) Experimental support for a model of birdsong production. Phys Rev E 68(4):41908. https://doi.org/10.1103/Physreve.68.041908
Mooney R (2000) Different subthreshold mechanisms underlie song selectivity in identified HVc neurons of the zebra finch. J Neurosci 20(14):5420–5436
Perl YS, Arneodo EM, Amador A, Goller F, Mindlin GB (2011) Reconstruction of physiological instructions from zebra finch song. Phys Rev E 84(5):051909. https://doi.org/10.1103/Physreve.84.051909
Prather JF, Peters S, Nowicki S, Mooney R (2008) Precise auditory-vocal mirroring in neurons for learned vocal communication. Nature 451(7176):305-U302. https://doi.org/10.1038/nature06492
Pressnitzer D, Bestel J, Fraysse B (2005) Music to electric ears: pitch and timbre perception by cochlear implant patients. Ann NY Acad Sci 1060(1):343–345
Quiroga RQ, Nadasdy Z, Ben-Shaul Y (2004) Unsupervised spike detection and sorting with wavelets and superparamagnetic clustering. Neural Comput 16(8):1661–1687
Riede T, Goller F (2010) Peripheral mechanisms for vocal production in birds—differences and similarities to human speech and singing. Brain Lang 115(1):69–80. https://doi.org/10.1016/j.bandl.2009.11.003
Riede T, Fisher JH, Goller F (2010) Sexual dimorphism of the zebra finch syrinx indicates adaptation for high fundamental frequencies in males. PLoS One 5(6):e11368. https://doi.org/10.1371/journal.pone.0011368
Ritschard M, Brumm H (2011) Effects of vocal learning, phonetics and inheritance on song amplitude in zebra finches. Anim Behav 82(6):1415–1422
Srivastava KH, Elemans CP, Sober SJ (2015) Multifunctional and context-dependent control of vocal acoustics by individual muscles. J Neurosci 35(42):14183–14194
Theunissen FE, Doupe AJ (1998) Temporal and spectral sensitivity of complex auditory neurons in the nucleus HVc of male zebra finches. J Neurosci 18(10):3786–3802
Theunissen FE, Sen K, Doupe AJ (2000) Spectral–temporal receptive fields of nonlinear auditory neurons obtained using natural sounds. J Neurosci 20(6):2315–2331
Theunissen FE, Amin N, Shaevitz SS, Woolley SMN, Fremouw T, Hauber ME (2004) Song selectivity in the song system and in the auditory forebrain. Ann Ny Acad Sci 1016:222–245. https://doi.org/10.1196/Annals.1298.023
Thorpe WH (1961) Bird-song: the biology of vocal communication and expression in birds. Cambridge University Press, Oxford
Town SM, Bizley JK (2013) Neural and behavioral investigations into timbre perception. Front Syst Neurosci 7
Volman S (1996) Quantitative assessment of song-selectivity in the zebra finch “high vocal center”. J Comp Physiol A 178(6):849–862
Acknowledgements
We thank Cecilia T. Herbert and Gabriel B. Mindlin for comments and discussions that greatly improved the manuscript. This work was partially supported by Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET, Argentina), Agencia Nacional de Promoción Científica y Tecnológica (ANCyT, Argentina), Universidad de Buenos Aires (UBA, Argentina) and National Institutes of Health (NIH, USA) through grant R01-DC-012859. Experimentation and surgical procedures were conducted following protocols approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Buenos Aires.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
359_2017_1238_MOESM1_ESM.pdf
Online Resource 2: MOD-to-BOS relative differences in spectral irregularity within each song syllable. In this figure, we show the temporal evolution of the spectral irregularity (IRR) as defined in the main text. Each subfigure (a-e) corresponds to the analysis for a different bird. IRR time traces are shown in the top panel, with black traces corresponding to BOS and red traces to MOD, respectively. The second panel shows the MOD-to-BOS relative difference in IRR, averaged over 5 ms windows. The two bottom panels show the BOS and MOD sound traces (arb. unit), color coded as above (sampling rate: 20 kHz). In the songs from all the birds present in our study, IRR is only slightly changed. Relative differences account for the similarity between the IRR of each syllable present in the BOS and MOD stimuli, as can be seen from the superimposing traces in each of the top panels in a-e and from the near-zero values for relative differences (second panel of a-e). Grouped data (f) shows a histogram for the relative difference values of the 22 syllables from the songs of the 5 birds present in this study (bin size = 0.005). From the 530 temporal bins analyzed, only a small percentage (2.8%) present a deviation larger than 3%. Distribution values are mostly within this range, and only a few rare cases present differences larger than 5% (0.4% of time bins). The small percentage of these occurrences and the fact that they are symmetrically distributed with respect to the peak at [-0.005,0.005] indicate that the induced changes in the creation of the MOD are subtle ones. Statistically, this distribution is indistinguishable from a zero-mean normal distribution (t-test, p=0.61). Comparable results are obtained for spectral centroid (see Online Resource 1) (PDF 2142 KB)
359_2017_1238_MOESM2_ESM.pdf
Online Resource 1: MOD-to-BOS relative differences in spectral centroid within each song syllable. In this figure, we show the temporal evolution of the spectral centroid (SC) as defined in the main text. Each subfigure (a-e) corresponds to the analysis for a different bird. SC time traces (in kHz) are shown in the top panel, with black traces corresponding to BOS and red traces to MOD, respectively. The second panel shows the MOD-to-BOS relative difference in SC, averaged over 5 ms windows. The two bottom panels show the BOS and MOD sound traces (arb. unit), color coded as above (sampling rate: 20 kHz). In the songs from all the birds present in our study, SC is only slightly changed. Relative differences account for the similarity between the SC of each syllable present in the BOS and MOD stimuli, as can be seen from the superimposing traces in each of the top panels in a-e and from the near-zero values for relative differences (second panel of a-e). Grouped data (f) shows a histogram for the relative difference values of the 22 syllables from the songs of the 5 birds present in this study (bin size = 0.005). From the 530 temporal bins analyzed, only a small percentage (2%) present a deviation larger than 3%. Distribution values are mostly within this range, and only a few rare cases present differences larger than 5% (0.2% of time bins). The small percentage of these occurrences and the fact that they are symmetrically distributed with respect to the peak at [-0.005,0.005] indicate that the induced changes in the creation of the MOD are subtle ones. Statistically, this distribution is indistinguishable from a zero-mean normal distribution (t-test, p=0.17). Comparable results are obtained for spectral irregularity (see Online Resource 2) (PDF 2152 KB)
Rights and permissions
About this article
Cite this article
Boari, S., Amador, A. Neural coding of sound envelope structure in songbirds. J Comp Physiol A 204, 285–294 (2018). https://doi.org/10.1007/s00359-017-1238-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00359-017-1238-9