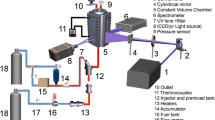
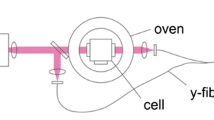
Abstract
This work identifies the fluorescence characteristics of a perfluorinated ketone, 2-trifluoromethyl-1,1,1,2,4,4,5,5,5-nonafluoro-3-pentanone, further referred to as fluoroketone. This compound is suitable for use with the third harmonic of an Nd:YAG laser for quantitative concentration measurements, as it exhibits strong emission even for relatively low excitation and has a near-linear response of fluorescence intensity with concentration. This makes it suitable for a broad range of fluorescence applications. The absorption cross-section of 3.81 × 10−19 cm2 was found to be constant for a temperature range of 293–441 K and a pressure range of 1–18 atm. A calibration line has been generated that relates the concentration of gaseous and liquid fluoroketone with its absorption coefficient.










Similar content being viewed by others
Abbreviations
- P :
-
Pressure (atm)
- T :
-
Temperature (K)
- P cr :
-
Critical pressure (atm)
- T cr :
-
Critical temperature (K)
- T r :
-
Reduced temperature
- P r :
-
Reduced pressure
- ρ :
-
Density (kg/m3)
- I :
-
Intensity of the laser sheet (J/m2)
- λ :
-
Wavelength of laser (nm)
- σ :
-
Absorption cross-section (cm2)
- σ g :
-
Absorption cross-section for the ground state (cm2)
- σ e :
-
Absorption cross-section for the excited state (cm2)
- N g :
-
Number of molecules in the ground state
- N e :
-
Number of molecules in the excited state
- N :
-
Total number of molecules
- dV :
-
Collection volume
- dl :
-
Differential length along the laser propagation direction
- A :
-
Area perpendicular to laser propagation direction
- N A :
-
Avogadro’s number
- M:
-
Molecular weight
- R :
-
Universal gas constant
- N ph :
-
Number of incident photons
- N fl :
-
Number of emitted photons due to fluorescence
- h :
-
Planck’s constant
- c :
-
Speed of light (m/s)
- φ :
-
Quantum yield of fluorescence
- α:
-
Absorption coefficient
- η optic :
-
Collection optics efficiency
- S :
-
Fluoroketone vapor fluorescence signal
- F, K :
-
Constants in the fluorescence equation
References
Crimaldi JP (2008) Planar laser induced fluorescence in aqueous flows. Exp Fluid 44:851–863
Frackowiak B, Strzelecki A, Lavergne G (2008) A liquid vapor interface positioning method applied to PLIF measurements around evaporating monodisperse droplet streams. Exp Fluid 46:671–682
Gustavsson JPR, Segal C (2007) Fluorescence spectrum of 2-trifluoromethyl-1,1,1,2,4,4,5,5,5,-nonafluoro-3-pentanone. Appl Spectrosc 61:903–907
Hanson RK, Mungal MG, Grisch F, Thurber MC, Smith SH, Hasselbrink EF (1996) Temperature and mixture fraction imaging of gaseous flows using acetone PLIF. In: 27th AIAA fluid dynamics conference, New Orleans, LA, 17–20 June 1996, AIAA 96–1964
Karasso PS, Mungal MG (1997) PLIF measurements in aqueous flows using the ND: YAG laser. Exp Fluid 23:382–387
Koch J, Hanson R (2003) Temperature and excitation wavelength dependencies of 3-pentanone absorption and fluorescence for PLIF applications. Appl Phys B 76:319–324
Melton LA, Lipp CW (2003) Criteria for quantitative PLIF experiments using high power lasers. Exp Fluid 35:310–316
Muhlfriedel K, Baumann KH (2000) Concentration measurements during mass transfer across liquid phase boundaries using planar laser induced fluorescence. Exp Fluid 28:279–281
Owens JG (2003) Understanding the stability and environmental characteristics of a sustainable Halon alternative. In: Proceedings of the 13th Halon Options technical working conference, Albuquerque, NM, 13–15 May 2003, NIST Special Publication 984-1
Roy A, Segal C (2009) Experimental study of subcritical to supercritical jet mixing. In: 47th AIAA aerospace sciences meeting and exhibit, Orlando, FL, 5–8 January 2009, AIAA 2009-809
Roy A, Segal C (2010) Sub-to-supercritical jet mixing and core length analysis. In: 48th AIAA aerospace sciences meeting and exhibit, Orlando, FL, 4–7 January 2010, AIAA 2010-1149
Segal C, Polikhov S (2008) Subcritical to supercritical mixing. Phys Fluid 20:052101
Thurber M, Hanson R (1999) Pressure and composition dependences of acetone laser-induced fluorescence with excitation at 248, 266, and 308 nm. Appl Phys B 69:229–240
Thurber M, Grisch F, Kirby B, Votsmeier M, Hanson R (1996) Measurements and modeling of acetone laser-induced fluorescence with implications for temperature imaging-diagnostics. Appl Opt 37:4963–4978
Tran T, Kochar Y, Seitzman J (2008) Acetone photophysics at near critical to supercritical conditions. In: 46th AIAA aerospace sciences meeting and exhibit, Reno, NV, 7–10 January 2008, AIAA 2008-204
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Roy, A., Gustavsson, J.P.R. & Segal, C. Spectroscopic properties of a perfluorinated ketone for PLIF applications. Exp Fluids 51, 1455–1463 (2011). https://doi.org/10.1007/s00348-011-1163-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00348-011-1163-6