Abstract
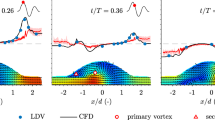
To discuss the validity of the hemodynamic hypothesis of aneurysm rupture, we used a patient-specific, realistic aneurysm model to reveal the flow structure and wall shear stress distribution in two cases: one with an unruptured aneurysm and the other with a ruptured aneurysm. We used particle imaging velocimetry and laser Doppler velocimetry to measure velocity profiles of intra-aneurysmal flow. Both cases had a circulating flow along the aneurysm wall, although the second case had a recirculating zone only in the minimum phase. Differences in the wall shear stress profile may identify aneurysm rupture.









Similar content being viewed by others
References
Adrian RJ (1986) Image shifting technique to resolve directional ambiguity in double-pulsed velocimetry. Appl Opt 25:3855–3858
Adrian RJ (1997) Dynamic ranges of velocity and spatial resolution of particle image velocimetry. Meas Sci Technol 8:1393–1398
Creasy JK, Crump DB, Knox L, Kerber CW, Price RR (1995) Design and evaluation of a flow phantom. Acad Radiol 2:902–904
Crompton MR (1966) Mechanism of growth and rupture in cerebral berry aneurysms. Brit Med J 1:1138–1142
Davis P (1995) Flow-mediated endothelial mechanotransduction. Physiol Rev 75:519–560
Ford MD, Nikolov HN, Milner JS, Lownie SP, Demont EM, Kalata W, Loth F, Holdsworth DW, Steinman DA (2008) PIV-measured versus CFD-predicted flow dynamics in anatomically realistic cerebral aneurysm models. J Biomech Eng 130:021015
Foutrakis GN, Yonas H, Sclabassi RJ (1999) Saccular aneurysm formation in curved and bifurcating arteries. Am J Neuroradiol 20:1309–1317
Gobin YP, Counord JL, Flaud P, Duffaux J (1994) In vitro study of haemodynamics in a giant saccular aneurysm model: Influence of flow dynamics in the parent vessel and effects of coil embolization. Neuroradiology 36:530–536
Hashimoto N, Handa H, Nagata I, Hazama F (1980) Experimentally induced cerebral aneurysms in rats: Part V. Relation of hemodynamics in the circle of Willis to formation of aneurysms. Surg Neurol 13:41–45
Hassler O (1972) Scanning electron microscopy of saccular intracranial aneurysms. Am J Pathol 68:511–519
Hennerici M, Rautenberg W, Sitzer G, Schwartz A (1987) Transcranial Doppler ultrasound for the assessment of intracranial arterial flow velocity Part 1. Surg Neurol 27:439–448
Hopkins LM, Kelly JT, Wexler AS, Prasad AK (2000) Particle image velocity measurements in complex geometries. Exp Fluids 29:91–95
International Study of Unruptured Intracranial Aneurysms Investigators (2003) Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet 362:103–110
Johnston SC, Gress DR, Kahn JG (1999) Which unruptured cerebral aneurysms should be treated? a cost-utility analysis. Neurology 52:1806–1815
Juvela S, Porras M, Heiskanen O (1993) Natural history of unruptured intracranial aneurysms: a long-term follow-up study. J Neurosurg 79:174–182
Kayembe KNT, Sasahara M, Hazama F (1984) Cerebral aneurysms and variations of the circle of Willis. Stroke 15:846–850
Kim SK, Chung SK (2004) An investigation on airflow in disordered nasal cavity and its corrected models by tomographic PIV. Meas Sci Technol 15:1090–1096
Ku DN, Giddens DP, Zarins CK, Glagov S (1985) Pulsatile flow and atherosclerosis in the human carotid bifurcation. Atherosclerosis 5:293–302
Liou TM, Chang WC, Liao CC (1997) Experimental study of steady and pulsatile flows in cerebral aneurysm model of various sizes at branching site. J Biomech Engr 119:325–332
Massoud TF, Hademenos GJ, Young WL, Gao E, Pile-Spellman J, Vinuela F (1998) Principles and philosophy of modeling in biomedical research. FASEB J 12:275–285
Roach MR, Scott S, Ferguson GG (1972) The hemodynamic importance of the geometry of bifurcation in the circle of Willis (glass model studies). Stroke 3:255–267
Sho E, Sho M, Singh TM, Nanjo H, Komatsu M, Xu C, Masuda H, Zarins CK (2002) Arterial enlargement in response to high flow requires early expression of matrix metalloproteinases to degrade extracellular matrix. Exp Mol Pathol 73:142–153
Shojima M, Oshima M, Takagi K, Torii R, Nagata K, Shirouzu I, Morita A, Kirino T (2005) Role of the bloodstream impacting force and the local pressure elevation in the rupture of cerebral aneurysms. Stroke 36:1933–1938
Stehbens WE (1989) Etiology of intracranial berry aneurysms. J Neurosurg 70:823–831
Stehbens WE, Liepsch DW, Poll A, Erhard W (1995) Recording of unexpectedly high frequency vibrations of blood vessel walls in experimental arteriovenous fistulae of rabbits using a laser vibrometer. Biorheology 32:631–641
Steiger HJ, Poll A, Liepsch D, Reulen HJ (1987) Basic flow structure in saccular aneurysms: a flow visualization study. Heart Vess 3:55–65
Steinman DA (2002) Image-based computational fluid dynamics modeling in realistic arterial geometries. Ann Biomed Engr 30:483–497
Suzuki J, Ohara H (1978) Clinicopathological study of cerebral aneurysms. J Neurosurg 48:505–514
Tateshima S, Murayama Y, Villablanca JP, Morino T, Takahashi H, Yamauchi T, Tanishita K, Vinuela F (2001) Intra-aneurysmal flow dynamics study featuring an acrylic aneurysm model manufactured using a computerized tomography angiogram as a mold. J Neurosurg 95:1020–1027
Tateshima S, Murayama Y, Villablanca JP, Morino T, Nomura K, Tanishita K, Vinuela F (2003a) In vitro measurement of fluid-induced wall shear stress in unruptured cerebral aneurysms harboring blebs. Stroke 34:187–192
Tateshima S, Vinueka F, Villablanca JP, Murayama Y, Morino T, Nomura K, Tanishita K (2003b) Three-dimensional blood flow analysis in a wide-necked internal carotid artery-ophthalmic artery aneurysm. J Neurosurg 99:526–533
Tateshima S, Grinstead J, Sinha S, Nien Y, Murayama Y, Villablanca JP, Tanishita K, Vinuela F (2004) Intra-aneurysmal flow visualization by phase contrast magnetic resonance imaging: feasibility in vitro study using a geometrically realistic aneurysm model. J Neurosurg 100:1041–1048
Tateshima S, Tanishita K, Omura H, Villablanca JP, Vinuela F (2007) Intra-aneurysmal hemodynamics during the growth of an unruptured aneurysm: in vitro study using longitudinal CT angiogram database. Am J Neuroradiol 28:622–627
Tateshima S, Tanishita K, Vinuela F (2008) Hemodynamics and cerebrovascular disease. Surg Neurol 70:447–453
Tognetti F, Limoni P, Testa C (1983) Aneurysm growth and hemodynamic stress. Surg Neurol 20:74–78
Tremmel M, Dhar S, Levy EI, Mocco J, Meng H (2009) Influence of intracranial aneurysm-to-parent vessel size ratio on hemodynamics and implication for rupture: results from a virtual experimental study. Neurosurgery 64:622–631
Tsutsumi K, Ueki K, Morita A et al (2000) Risk of rupture from incidental cerebral aneurysms. J Neurosurg 93:550–553
Ujiie H, Tachibana H, Hiramatsu O, Hazel AL, Matsumoto T, Ogasawara Y, Nakajima H, Hori T, Takakura K, Kajiya F (1999) Effects of size and shape (aspect ratio) on the hemodynamics of saccular aneurysms: a possible index for surgical treatment of intracranial aneurysms. Neurosurgery 45:119–129
Ujiie H, Tamano Y, Sasaki K, Hori T (2001) Is the aspect ratio a reliable index for predicting the rupture of a saccular aneurysm? Neurosurgery 48:495–502
Weir B, MacDonald RL (1996) Aneurysms and subarachnoid hemorrhage. In: Wilkins RH, Rengachary SS (eds) Neurosurgery. McGraw-Hill, New York, pp 2191–2213
Wiebers DO, Whisnant JP, O’Fallon WM (1981) The natural history of unruptured intracranial aneurysms. N Engl J Med 304:696–698
Acknowledgments
This work was partially supported by a grant-in-aid for scientific research (A 17206020) provided by the Japan Society for the Promotion of Science. The authors thank Ms. Kiyoe Nomura for helpful discussions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Morino, T., Tanoue, T., Tateshima, S. et al. Intra-aneurysmal blood flow based on patient-specific CT angiogram. Exp Fluids 49, 485–496 (2010). https://doi.org/10.1007/s00348-009-0816-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00348-009-0816-1