Abstract
Purpose
Platelet-rich plasma (PRP) as a regenerative therapy has gained interest in the field of andrology for the treatment of erectile dysfunction (ED) and Peyronie’s disease (PD). This systematic review aims to critically evaluate the current evidence on the use of PRP for these conditions.
Methods
We performed a systematic literature search according to the PRISMA guidelines using PubMed and Scopus databases in December 2023. Studies were included if they evaluated the effect of PRP therapy for ED or PD in humans.
Results
We identified 164 articles, 17 of which were included, consisting of 11 studies on ED, 5 studies on PD, and 1 study on both. We included four randomized controlled trials, 11 prospective cohort studies, and three retrospective cohort studies including a total of 1099 patients. The studies on ED and PD generally showed small to moderate benefits with mild and transient side effects and no major adverse events were reported. General limitations included variations in PRP protocols, small sample sizes, short follow-up periods, and lack of control groups except in the three randomized trials on ED and the one on PD.
Conclusion
The literature on PRP therapy in andrology is limited and difficult to interpret due to variations in protocols and methodological drawbacks. Further research is necessary to determine the optimal preparation and treatment protocols for PRP therapy and clarify its effectiveness in andrology.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Platelet-rich plasma (PRP) refers to the liquid fraction of peripheral blood, which has been processed to ensure a high concentration of platelets [1]. Platelets have a crucial role in the aggregation process and promote coagulation through adhesion, activation, and aggregation processes [2]. However, recent studies revealed a broader perspective on platelets and their functions. Since the platelets are rich in growth factors (GF), PRP preparations are believed to have potential in regenerative medicine [3]. The main GFs released by platelets in an inflammatory environment are platelet-derived GF (PDGF), fibroblast GF (FGF), epidermal GF (EGF), insulin-like GF (IGF) as well some interleukin (IL) [4]. In PRP therapy, a blood sample is collected and centrifuged to isolate the relevant fraction. This is then injected into the tissue where an effect is desired. PRP therapy has been used in various conditions, such as musculoskeletal injuries, wound healing, and dermatological disorders [1]. PRP appeared to demonstrate mitogenic, chemotactic, and angiogenic properties, rooted in its ability to induce soft tissue proliferation and collagen deposition by activating fibroblasts [5]. Recently, PRP therapy has also gained increasing interest in the field of andrology for the management of erectile dysfunction (ED) and Peyronie's disease (PD) [6]. According to the literature, PRP has demonstrated neurotrophic effects on damaged nerves. Animal studies focusing on erectile function in male rats with cavernous nerve injuries have reported improved erections following PRP therapy compared to the injured control group [7, 8]. Notably, PRP may enhance axon myelination, reduce apoptosis, and facilitate fiber regeneration. However, due to a paucity of robust clinical studies, there are no official recommendations for the use of PRP in these conditions and the guidelines of the European Association of Urology (EAU) or American Association of Urology (AUA) specifically denotes it as experimental in ED and PD [9]. In this systematic review, we aim to provide an overview of the current evidence on the use of PRP therapy in the management of ED and PD. We will review the available clinical studies, discuss the potential mechanisms of action of PRP, and highlight the limitations and future directions of this therapy in andrology.
Methods
Evidence acquisition
We registered the protocol in the PROSPERO database (ID CRD42024495624) and reported according to the PRISMA guidelines [10].
Search strategy
Two authors (M.G.A. and E.D.) conducted a comprehensive bibliographic search on MEDLINE and Scopus on January 16th, 2024 to identify studies published since 1995 describing the benefits of PRP therapy in the management of ED and PD. The following search strings were used:
-
Erectile Dysfunction: ("erectile dysfunction" OR "sexual dysfunction" OR "impotence") AND ("platelet rich plasma" OR "PRP")
-
Peyronie’s Disease: (“peyronie’s disease” OR “penile curvature” OR “penile induration”) AND (“platelet rich plasma” OR “PRP”)
-
(TITLE-ABS-KEY (erectile dysfunction OR sexual dysfunction OR impotence) AND TITLE-ABS-KEY (platelet rich plasma OR PRP) AND (LIMIT-TO (DOCTYPE, "ar")))
-
(TITLE-ABS-KEY ((peyronie’s disease OR penile curvature OR penile induration) AND TITLE-ABS-KEY (platelet rich plasma OR PRP) AND (LIMIT-TO (DOCTYPE, "ar")))
Study selection
We used the Population, Intervention, Comparator, Outcome, Study (PICOS) model to define study eligibility [11]. PICOS criteria were set as follows: Population—patients affected by ED or PD; Intervention—autologous PRP injection; Comparator—Human patients affected by ED or PD receiving other types of treatments or no treatments at all; Outcome—variations of degree of penile curvature or erectile function in terms of IIEF, IIEF-5, IIEF-EF, ED duration, Erection Hardness Score [EHS], SEP [Sexual Encounter Profile], end-diastolic velocity [EDV], peak systolic velocity [PSV], resistive index [RI] or arterial diameter. Study—retrospective and prospective studies (Supplementary Table 1). No minimum number on patient population was applied.
Only English-language articles were considered for inclusion, with case reports, review articles, and publications with missing full texts (abstracts only) being excluded. Only studies conducted on human patients were included. Additional references were sought by hand-searching the reference lists of included studies and identified review papers. Due to recognized discrepancies in methodology, the decision was made to describe individual studies without conducting meta-analyses.
Data extraction
We recorded the following items: first author, study design, sample size, baseline parameters and post-treatment parameters (patients’ age, IIEF, IIEF 5, IIEF-EF, degree of curvature, ED duration, EHS, SEP, EDV, PSV, [RI or arterial diameter], type of treatment, treatment dose and number of doses. Discrepancies in study selection were resolved by consensus with the coauthors. Statistical analyses were not performed.
Risk of bias
Two authors (M.G.A and E.D.) assessed the risk of bias and discrepancies were resolved by consensus with the coauthors. We employed the Cochrane Collaboration's Risk of Bias tool to evaluate the risk of bias in randomized trials [12]. This involved assessing criteria such as random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other potential biases. In the case of comparative non-randomized studies, we utilized the Risk of Bias in Non-randomized Studies of Interventions (ROBINS-I) tool, evaluating criteria such as confounding, participant selection, intervention measurement, deviations from intended interventions, missing data, outcome measurement, selection of reported results, and overall risk of bias [13]. For single-arm studies, we evaluated the risk of bias using the criteria recommended by the European Association of Urology Guidelines Office, covering aspects like a priori protocol, participant selection, adequate handling of missing data, specification of outcomes/selective reporting, and measurement of outcomes.
Evidence synthesis
Description of the studies included
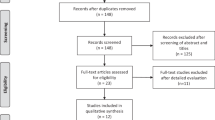
A total of 164 records were identified. After duplicates removal, 119 papers were subdued to titles screening. The remaining 33 abstracts were screened, and after excluding 16 records, a total of 17 papers were deemed eligible for review (Fig. 1). All clinical studies used autologous PRP but both preparation and injection methods differed across studies as specified in Tables 1 and 2.
Flow diagram of literature search and study selection. *Consider, if feasible to do so, reporting the number of records identified from each database or register searched (rather than the total number across all databases/registers). **If automation tools were used, indicate how many records were excluded by a human and how many were excluded by automation tools.From: Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. https://doi.org/10.1136/bmj.n71. For more information, visit: http://www.prisma-statement.org/
Seventeen studies (four Randomized Controlled Trials [RCT], 11 prospective cohort studies and two retrospective cohort studies including a total of 1099 patients) described the outcomes in patients receiving PRP injection therapy as a therapy for ED or PD. One of the studies included both ED and PD patients. Supplementary Figs. 1–6 illustrate the risk of bias of the included studies, with the majority of studies deemed at high risk of bias.
PRP and erectile dysfunction
Twelve publications regarding ED were identified, including two retrospective case series, seven prospective uncontrolled trials, and three randomized controlled trials (Table 1). All studies evaluated erectile function improvements using either the 5-item or the erectile function domain of the International Index of Erectile Function questionnaire (IIEF-5 and IIEF-EF, respectively) [14, 15]. Four studies also used penile duplex ultrasound to investigate end-diastolic velocity (EDV), peak systolic velocity (PSV), resistivity index (RI) and mean artery diameter [16,17,18]. Side effects were generally limited and included mild pain, bruising and development of a small fibrotic plaque in a single patient [19]. No major adverse events were reported in any of the studies.
The first identified study was a retrospective trial published by Matz et al. in 2018 [20]. It included 17 patients, 5 of whom suffered from organic ED. After initial preparation of autologous blood, the authors added a calcium chloride solution, converting fibrinogen to fibrin to create what they termed platelet-rich fibrin matrix. This may bind platelets at the site of injection for a longer time but is still considered a form of PRP. The patients received 1 to 8 injections upon their own request and were followed up at various timepoints. After a mean time of 15.5 months, the IIEF-5 scores improved by an average of 4.14 points (no p-value given). However, the completeness of follow-up was unclear, and the visits were guided by the subjective response of the patients likely skewing the results toward an increasing function score. Additionally, there was no mention of concurrent ED treatments, and no absolute IIEF-5 values were mentioned for any time-point. In the second retrospective study, Geyik et al. compared the effectiveness of three PRP injections combined with low-intensity shock wave therapy (Li-SWT) to that of Li-SWT alone in 184 PDE5-I non-responders [21]. All patients were allowed to continue using PDE5-Is and after 6 months the IIEF-EF score increased from 14.33 ± 4.39 to 23.8 ± 4.37 (p < 0.001) for the Li-SWT only group, while it increased from 17.82 ± 3.44 to 26.3 ± 2.55 (p < 0.001) in the combination group. No statistically significant differences between the groups were reported, suggesting no added effect of PRP. However, these results should be interpreted with caution as the two groups were not comparable regarding baseline characteristics and the selection criteria for choice of treatment were unclear. Further, it is unclear if the study looked at consecutive patients or if some treated men had been lost to follow-up.
In a more structured study, Tas et al. prospectively evaluated 31 treatment naïve ED patients with metabolic syndrome for 6 months following three PRP injections [19]. Here, the median IIEF-EF score increased from 18 to 20. While this change was statistically significant, the low magnitude of change indicated a lack of a clinically meaningful effect. A similar study by Wong et al. (n = 30) found an increase in the mean IIEF-5 score from 12.03 ± 5.10 at baseline to 16.59 ± 5.5 two weeks after the third PRP injection (p < 0.001) [22]. While the concurrent use of PDE5-Is in most of the participants represents a potential confounder, an increase of this size does meet the minimal requirement for a clinically relevant change. The prospective interventional cohort study conducted by Sajjad et al. compared the efficacy of Li-SWT and PRP in a cohort of 60 patients affected by ED [23]. Patients were non-randomly assigned to the PRP group, receiving multiple-sites weekly injections for a 6-week-period, or the Li-SWT group, receiving 300 shocks twice a week for a total of 6 weeks. Even though a high percentage of positive results have been reported by both groups, the mean IIEF-5 score improvement at 12-weeks follow up was not statistically significant (p > 0.005) [23]. Two more prospective case series were conducted by Zaghloul et al. [17, 18]. In both studies, PDE5-I non-responders were prescribed a daily dose of 5 mg tadalafil and 20 mg vardenafil on demand and subjected to a PRP injections. In the first study (n = 34) the mean IIEF-5 score increased from 7.7 ± 2.7 to 13.2 ± 6.8 (p < 0.001) at 3 months follow-up, while no statistically significant changes in penile duplex ultrasound parameters were observed [17]. In the second study an increase in the mean IIEF-5 score from 8.04 ± 2.7 to 12.1 ± 5.6 (p = 0.003) was observed in diabetic men (n = 24) and an improvement from 10.2 ± 0.9 to 14.8 ± 4.8 (p = 0.001) in non-diabetic men (n = 24) [18]. EDV and PSV also increased at 3 months follow-up. Although these results seem encouraging, it must be stressed that the very high dosing of PDE5-Is is likely to account for at least part of the improvement as it represents a 50% increase from the previous dosing of the participants [18]. A study conducted by Schirmann et al. enrolled 15 patients affected by vascular ED who had not responded to previous treatments [24]. According to the protocol, in each of the three sessions conducted 15 days apart, the patients received a 3 mL injection of PRP combined with sodium citrate in each corpus cavernosum, along with a 6 mL subcutaneous injection. A statistically significant improvement in the IIEF-EF score was observed during follow-ups, with the score rising from 11.80 ± 5.51 at baseline to 16.80 ± 4.97 (p = 0.001) at the one-month follow-up, 16.23 ± 5.10 (p = 0.003) at the three-month follow-up, and 15.15 ± 6.44 (p = 0.02) at the six-month follow-up. The improvement in sexual discomfort score was evident only at the one-month follow-up (6.67 ± 19.97 vs. 16.67 ± 24.40; p = 0.043). However, it is noteworthy that the EHS and SEP did not exhibit improvement following the treatment. [24].
The prospective non-randomized cohort study conducted by Francomano et al. aimed to assess the response to PDE5-Is before and after PRP injection in a cohort of 150 vasculogenic ED patients [25]. A 5 mL PRP solution was injected into two sites in each corpora cavernosa, and no serious adverse events were reported. At the 1-month follow-up, nearly all patients (80%) resumed sexual activity and reported an improvement in the IIEF-5 score after using PDE5-Is, along with enhanced cavernosal blood flow following pharmacological stimulation. The results showed a significant increase in IIEF-5 scores from 12 ± 2.6 at baseline to 19 ± 3.0 after treatment (p < 0.0001) and a rise in PSV from 32 ± 5.5 cm/s before treatment to 42 ± 7.6 cm/s after treatment (p < 0.0001). Additionally, the study proposed that mean platelet volume (MPV) at baseline could serve as a predictive biomarker for PRP treatment outcomes, with lower MPV values associated with a higher likelihood of treatment response [25]. The final studies on ED and PRP are randomized, double-blind, placebo-controlled trials. Poulios et al. randomized men with mild or moderate vasculogenic ED to receive two PRP (n = 30) or placebo (n = 30) injections [26]. The effect was evaluated based on a minimal clinically important difference (MCID) defined as an improvement in the IIEF-EF score by 2 or more points for mild or mild to moderate ED or 5 or more points for moderate ED. At 1-, 3-, and 6-months follow-up, 22/29 (76%), 20/29 (69%), and 20/29 (69%) patients in the PRP group achieved the MCID, respectively. The corresponding numbers in the placebo group was 7/28 (25%), 10/26 (39%), and 7/26 (27%) with statistically significant between-group differences at all time points. In the second randomized trial, Shaher et al. also divided men with vasculogenic ED between PRP (n = 50) and placebo (n = 50) groups, using the same MCID definition as Poulios et al. [16, 26]. At 1-, 3-, and 6-months follow-up, 38/50 (76%), 36/50 (72%), and 35/50 (70%) patients in the PRP group achieved the MCID, compared to 9/50 (18%), 8/50 (16%), and 8/50 (16%) in the placebo group (p < 0.001 for all time points). The authors also observed improvements in penile blood flow on duplex ultrasound in the PRP group. While these studies do indicate a possible short-term effect of PRP, it is important to highlight that both studies find relatively small mean changes in the IIEF-EF scores of treated patients (3.3 and 4 points, respectively) and a surprising lack of overall placebo effects. Additionally, only the Poulios trial appears well designed with clear description of the blinding and randomization procedures and prohibition of PDE5-I use throughout the study. Thus, the study by Shaher et al. is severely hampered by methodological drawbacks as it makes no mention of concurrent ED treatments, fails to perform any statistical comparisons between the overall IIEF-EF scores in the PRP and placebo groups, and was not pre-registered in any form. In the third randomized controlled trial, Masterson et al. allocated a cohort of organic ED patients into PRP (n = 28) and saline solution (n = 33) groups [27]. The authors employed the same MCID definition used in the two aforementioned studies [16, 26]. At the 1-month follow-up, 58.3% (14/24) in the PRP group vs. 53.6% (15/28) in the placebo group achieved MCID (p = 0.730). Statistically significant changes in the mean IIEF-EF score from baseline were observed in both groups at 1 month (mean increase of 3.7 vs. 3.1; p = 0.026 vs. p = 0.009) and at 6 months (mean increase of 5 vs. 2.2; p = 0.003 vs. p = 0.045), but no statistically significant differences were noted between the two groups (p = 0.765 and p = 0.116). The increase in IIEF-EF at 3 months was not statistically significant, and there was no significant difference between the two subsets (p = 0.662). Despite efforts to reduce methodological drawbacks and bias through a double-blinded design, several limitations were acknowledged, including a small sample size, a high dropout rate, a short follow-up time, and differences in patient populations; furthermore, the authors reported a higher prevalence of patients with prediabetes in the control group [27].
PRP and Peyronie’s disease
The literature search revealed four prospective cohort studies, one retrospective case series (also included for ED), and a publication with preliminary results from a placebo controlled randomized trial examining PRP as a therapeutic intervention for PD (Table 2). The PRP injections were administered intralesional in all studies and side effects were generally minor and self-limiting including bruising, hematoma, ecchymosis, and mild pain. Only one case of skin infection was reported during a trial creating multiple channels along the entire longitudinal axis of the plaque [28]. Across the studies a single patient was reported to discontinue PRP treatment after four injections due to PD aggravation [29].
The first study was published by Virag et al. in 2017 [29]. This was a prospective cohort study investigating a combined injection of PRP and hyaluronic acid in 90 participants with established penile plaques and deformity. The authors used initial plaque needle fracturing and subsequent injections were performed upon patient request, with a reported average of 7.09 injections per patient. After the treatments a mean angle reduction of 16.54 degrees corresponding to a ~ 40% reduction in curvature (mean curvature before injection of 44.37 degrees ± 15.93 degrees) was observed. A potential issue in this regard is that the maximum curvature was reportedly measured on photographs of fully erected penises although approximately 1/3 of the participants in the study had ED. The objective improvements were accompanied by statistically significant reductions across the three domains of the Peyronie’s Disease Questionnaire and on subjective assessment 67.8% felt that the treatment had improved their initial condition.
In another prospective cohort study, conducted by Achraf et al., 65 patients affected by stable symptomatic Peyronie's Disease (PD) were enrolled [30]. These patients had not undergone any previous PD treatments. The cohort was subsequently divided into two groups based on the degree of penile curvature: 25°–35° in group 1 and 35°–45° in group 2. Each patient underwent 8 mL of PRP at each session, with an average of 6.1 injections in total. The findings revealed a significant improvement in penile curvature, reduction in pain, and enhanced erectile function following PRP injections. Specifically, a mean curvature reduction of 16.88° and 17.27° was noted in groups 1 and 2, respectively. Pain during sexual intercourse decreased (VAS: -34% in group 1 and -39% in group 2), and erectile function showed improvement as assessed by the IIEF questionnaire (+ 50% in group 1 vs. + 61% in group 2) [30]. The prospective cohort study published in 2023 by Zugail et al., enrolled 54 patients affected by stable PD who reported difficulties to perform coitus due to the disease [28]. The main procedure involved percutaneous needle tunnelling by creating multiple channels along the longitudinal axis of the plaque; then, 5–6 mL of PRP were injected, and 14 days after, daily use of vacuum device was initiated. Despite promising results being reported, with an improvement in penile curvature from 53.98 ± 23.19° at baseline to 30.09 ± 20.61° post-treatment (p = 0.001), it is impossible to establish if the benefits came from PRP injections or vacuum therapy. Analogously Alshuaibi et al. also applied a combined treatment approach using percutaneous needle tunnelling followed by penile modeling and PRP injection [28, 31]. However, in contrast to the aforementioned protocols, patients received the treatment after artificially induced erections and during general anesthesia; these measures could ensure a more accurate assessment of penile curvature and plaques position. Results from the cohort of 36 patients suggest a statistically significant improvement in penile curvature, with a mean improvement difference of 16.85 ± 14.81 (pretreatment mean curvature 57.5 ± 20.61 vs 40.86 ± 25.13 after treatment; p = 0.0001). Unfortunately, these promising outcomes are limited by assessment of penile curvature improvement by photographs taken by the patients and the lack of validated questionnaires for ED and PD evaluation. The retrospective case series was the one by Matz et al. described previously, in which the authors used a version of PRP termed platelet-rich fibrin matrix [20]. Twelve of the patients enrolled in this study suffered from PD and three of them underwent needle fracture of their plaques prior to PRP injections. However, no further details regarding the patients were reported and the paper simply states that 4/5 patients with available follow-up data reported subjective curvature improvement. The last paper on PRP and PD presents preliminary data from a randomized placebo-controlled crossover trial with an inclusion target of 80 men [32]. In this trial participants are randomized to either two intralesional PRP injections or two saline injections with planned cross over after 3 months. At the time of publication, 3 months follow-up data were available for 17 men and showed no changes in penile curvature in either group compared to baseline.
Discussion
No standardized PRP preparation methods or treatment protocols exist, and the treatment is in its infancy in the field of andrology. This makes it difficult to compare results across studies and to make definite conclusions regarding efficacy. Specifically there is a lack of a defined protocol for PRP preparation, agreement on injections timing or even the dose to administer and PRP therapy is not recommended by neither the EAU nor AUA guidelines. Thus, the treatment still represents an off-label therapy, and it is mainly applied in clinical trials. However, PRP is already being promoted as a curative treatment for sexual dysfunctions and is being offered to patients [6]. Therefore, it is important for clinicians dealing with these issues to be aware of the available data.
The main strength of our review is the inclusion of all clinical studies on PRP in the treatment of ED and PD including in dept descriptions of individual trials. However, the number of original studies is limited, and the individual trials contain important drawbacks, which limits our ability to draw firm conclusions. Overall, the literature on PRP is scarce with only few studies identified. The trials consistently show that the treatment is safe and no patients in any of the studies have experienced severe side effects after injections. The studies we selected are challenging to compare due to the heterogeneity of the cohort analyzed, as well as the barely defined inclusion and exclusion criteria. Indeed, studies enrolling patients affected by ED generally focus on vasculogenic or organic ED when specified. However, in some cases, exclusion criteria are not very restrictive, and iatrogenic ED patients are included as well [25]. Only one of the studies enrolling patients affected by PD specified the disease status [30]. In several studies positive effects of the treatment were reported and the use of validated questionnaires and scales provides some merit to these findings. However, most of the clinical trials are limited by their observational designs, small sample sizes, and short duration of follow-up. Notably, only four studies were randomized [16, 26, 27, 32] and two were case–control studies [21, 23]. In some cases, there was even a lack of a standardized treatment protocol within the individual study. The lack of control groups is especially problematic in conditions such as ED and PD because of the important psychological components when dealing with sexual dysfunction. Thus, it is well documented that placebo medications have a measurable effect in ED patients and there are several plausible biological explanations to explain this including changes in arousal and activation of central dopaminergic pathways [33]. Likewise, well designed clinical studies in PD patients have shown placebo effects on both objective and subjective parameters and bother from the condition may subside over time in some men even without treatment [34, 35].
For ED, we only identified one well designed and adequately reported randomized controlled trial [26]. This study showed benefits of PRP compared to placebo injections with potentially clinically relevant responses in a total of 20 men. However, the study definition of a MCID for the IIEF-EF questionnaire of 2 points in men with mild to moderate ED is questionable since this group was actually not specifically described when MCID values were determined for the questionnaire [36]. As this sub-group of men comprised most study participants it seems reasonable to remain somewhat skeptical of the clinical benefits. This is of particular importance as the established overall MCID for IIEF-EF is 4 points, while the study found a mean improvement of 3.3 points. Finally, it warrants mentioned that similar improvements have previously been noted in purely placebo treated patients and that the magnitude of the improvements is approximately 10 points on the IIEF-EF for PDE5-Is [37, 38].
For PD, no controlled studies were available and only one completed trial and two prospective cohort studies provided objective curvature measurements [28,29,30]. In the trial, the combination of PRP and hyaluronic acid injections looked promising with an improvement of a similar magnitude as what has been reported for collagenase injections [34]. However, although very preliminary, the early results from the ongoing randomized trial hints that the benefits may not be reproducible under controlled conditions. Additionally, formal comparisons of outcomes proved challenging due to the variety in follow-up length, typically between 1 and 12 months, as well as the diverse doses and number of PRP injections, varying from 0.5 to 9 mL and from 2 to 6 injections. The lack of agreement also applies to the preparation methods. In fact, the methods for preparing PRP differ in the inclusion of other substances such as calcium chloride, activation factors, and hyaluronic acid, as well as in centrifugation time and number of spins. As previously reported in studies, the concentration of growth factors in PRP preparation seems to vary among patients with ED. Therefore, this might represent an additional complication when comparing results between different studies and patients [39].
Future directions
One of the primary concerns when evaluating the advantages of PRP is establishing a validated and widely recognized preparation protocol. Standardization is crucial for determining the right dosage, the frequency of injections, the injection locations, potential dilutions with other substances, the number of centrifuge spins, and the duration of centrifugation. Although numerous PRP preparation protocols exist, each asserting its superiority, it would be prudent to standardize individual protocols, considering factors such as cost-effectiveness and their suitability for clinical settings [40]. The matter is further complicated by the finding that PRP growth factors may vary among men [39]. Theoretically this could mean that men with more severe disease may benefit less from PRP but further studies are needed to clarify this. Regarding potential future applications in the andrology field, PRP has not yet been extensively investigated in clinical trials related to male infertility. Nevertheless, in vitro studies have demonstrated encouraging outcomes, showing promising effects of PRP on semen quality and reducing oxidative stress. [41]. It has the potential to facilitate the proliferation of spermatogonial stem cells (SSC) and offer protection to semen samples during cryopreservation. [42,43,44]. Further trials are needed to assess if this will translate into clinical benefits.
Conclusion
The existing literature on PRP therapy in andrology remains limited. Trials confirm the procedure's safety, noting only minor and temporary adverse events and most seem to indicate small to moderate positive effects on outcomes for both ED and PD. Nevertheless, caution is warranted when interpreting these findings. Limitations include variations in PRP protocols and several methodological drawbacks. Future research is required to determine the optimal preparation and treatment protocols for PRP therapy, as well as to clarify its effectiveness in andrology.
Data availability
All data used in the study are avaliable in the original published trials referenced in the paper.
References
Everts P, Onishi K, Jayaram P, Lana JF, Mautner K (2020) Platelet-rich plasma: new performance understandings and therapeutic considerations in 2020. Int J Mol Sci 21:7794. https://doi.org/10.3390/ijms21207794
Alves R, Grimalt R (2018) A review of platelet-rich plasma: history, biology, mechanism of action, and classification. Skin Appendage Disord 4:18–24. https://doi.org/10.1159/000477353
Oudelaar BW, Peerbooms JC, Huisin’t Veld R, Vochteloo AJH (2019) Concentrations of blood components in commercial platelet-rich plasma separation systems: a review of the literature. Am J Sports Med 47:479–487. https://doi.org/10.1177/0363546517746112
Andia I, Abate M (2013) Platelet-rich plasma: underlying biology and clinical correlates. Regener Med 8:645–658. https://doi.org/10.2217/rme.13.59
McCormick SA (2012) Induction of dermal collagenesis, angiogenesis, and adipogenesis in human skin by injection of platelet-rich fibrin matrix. Arch Facial Plast Surg 14:132. https://doi.org/10.1001/archfacial.2011.784
Scott S, Roberts M, Chung E (2019) Platelet-rich plasma and treatment of erectile dysfunction: critical review of literature and global trends in platelet-rich plasma clinics. Sex Med Rev 7:306–312. https://doi.org/10.1016/j.sxmr.2018.12.006
Ding X-G, Li S-W, Zheng X-M, Hu L-Q, Hu W-L, Luo Y (2009) The effect of platelet-rich plasma on cavernous nerve regeneration in a rat model. Asian J Androl 11:215–221. https://doi.org/10.1038/aja.2008.37
Wu C, Wu Y, Ho H, Chen K, Sheu M, Chiang H (2012) The neuroprotective effect of platelet-rich plasma on erectile function in bilateral cavernous nerve injury rat model. J Sex Med 9:2838–2848. https://doi.org/10.1111/j.1743-6109.2012.02881.x
Salonia A MSBCCPCGHG (2023) EAU guidelines on sexual and reproductive health 2023 https://uroweb.org/guidelines/sexual-and-reproductive-health/chapter/citation-information (accessed 24 Apr 2023)
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA et al (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med 151:W65-94. https://doi.org/10.7326/0003-4819-151-4-200908180-00136
Schardt C, Adams MB, Owens T, Keitz S, Fontelo P (2007) Utilization of the PICO framework to improve searching PubMed for clinical questions. BMC Med Inform Decis Mak 7:16. https://doi.org/10.1186/1472-6947-7-16
Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD et al (2011) The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343:d5928. https://doi.org/10.1136/bmj.d5928
Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M et al (2016) ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 355:i4919. https://doi.org/10.1136/bmj.i4919
Rosen RC, Riley A, Wagner G, Osterloh IH, Kirkpatrick J, Mishra A (1997) The international index of erectile function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology 49:822–830. https://doi.org/10.1016/S0090-4295(97)00238-0
Rosen R, Cappelleri J, Smith M, Lipsky J, Peña B (1999) Development and evaluation of an abridged, 5-item version of the International Index of Erectile Function (IIEF-5) as a diagnostic tool for erectile dysfunction. Int J Impot Res 11:319–326. https://doi.org/10.1038/sj.ijir.3900472
Shaher H, Fathi A, Elbashir S, Abdelbaki SA, Soliman T (2023) Is platelet rich plasma safe and effective in treatment of erectile dysfunction? Random Control Study Urol 175:114–119. https://doi.org/10.1016/j.urology.2023.01.028
Zaghloul AS, Mahmoud ElNashar AER, GamalEl Din SF, Zaki Said S, Saad HM, Refaat Eldebs H et al (2021) Smoking status and the baseline international index of erectile function score can predict satisfactory response to platelet-rich plasma in patients with erectile dysfunction: a prospective pilot study. Andrologia. https://doi.org/10.1111/and.14162
Zaghloul AS, El-Nashaar AM, Said SZ, Osman IA, Mostafa T (2022) Assessment of the intracavernosal injection platelet-rich plasma in addition to daily oral tadalafil intake in diabetic patients with erectile dysfunction non-responding to on-demand oral PDE5 inhibitors. Andrologia. https://doi.org/10.1111/and.14421
Taş T, Çakıroğlu B, Arda E, Onuk Ö, Nuhoğlu B (2021) Early clinical results of the tolerability, safety, and efficacy of autologous platelet-rich plasma administration in erectile dysfunction. Sex Med 9:100313–100313. https://doi.org/10.1016/j.esxm.2020.100313
Matz EL, Pearlman AM, Terlecki RP (2018) Safety and feasibility of platelet rich fibrin matrix injections for treatment of common urologic conditions. Investig Clin Urol 59:61. https://doi.org/10.4111/icu.2018.59.1.61
Geyik S (2021) Comparison of the efficacy of low-intensity shock wave therapy and its combination with platelet-rich plasma in patients with erectile dysfunction. Andrologia. https://doi.org/10.1111/and.14197
Wong S-M, Chiang B-J, Chen H-C, Wu Y-N, Lin Y-H, Liao C-H (2021) A short term follow up for intracavernosal injection of platelet rich plasma for the treatment of erectile dysfunction. Urol Sci 32:171. https://doi.org/10.4103/UROS.UROS_22_21
Sajjad K, Sohail M, Momin HA, Shafique RA, Nazir M, Ahmad S et al (2021) Effect of low-energy shockwave therapy versus platelets rich plasma therapy in patients with erectile dysfunction. J Pharm Res Int 2021:168–172. https://doi.org/10.9734/jpri/2021/v33i32A31730
Schirmann A, Boutin E, Faix A, Yiou R (2022) Pilot study of intra-cavernous injections of platelet-rich plasma (P-shot®) in the treatment of vascular erectile dysfunction. Prog Urol 32:1440–1445. https://doi.org/10.1016/j.purol.2022.05.002
Francomano D, Iuliano S, Dehò F, Capogrosso P, Tuzzolo P, La Vignera S et al (2023) Regenerative treatment with platelet-rich plasma in patients with refractory erectile dysfunction: short-term outcomes and predictive value of mean platelet volume. Minerva Endocrinol. https://doi.org/10.23736/S2724-6507.23.04060-5
Poulios E, Mykoniatis I, Pyrgidis N, Zilotis F, Kapoteli P, Kotsiris D et al (2021) Platelet-rich plasma (PRP) improves erectile function: a double-blind, randomized placebo-controlled clinical. Trial J Sex Med 18:926–935. https://doi.org/10.1016/j.jsxm.2021.03.008
Masterson TA, Molina M, Ledesma B, Zucker I, Saltzman R, Ibrahim E et al (2023) Platelet-rich plasma for the treatment of erectile dysfunction: a prospective, randomized, double-blind placebo-controlled clinical Trial. J Urol 210:154–161. https://doi.org/10.1097/JU.0000000000003481
Zugail AS, Alshuaibi M, Lombion S, Beley S (2023) Safety and feasibility of percutaneous needle tunneling with platelet-rich plasma injections for Peyronie’s disease in the outpatient setting: a pilot study. Int J Impot Res. https://doi.org/10.1038/s41443-023-00744-y
Virag R, Sussman H, Lambion S, de Fourmestraux V (2017) Evaluation of the benefit of using a combination of autologous platelet rich-plasma and hyaluronic acid for the treatment of Peyronie’s disease. Sexual Health Issues. https://doi.org/10.15761/SHI.1000102
Achraf C, Abdelghani PA, Jihad PEA (2023) Platelet-rich plasma in patients affected with Peyronie’s disease. Arab J Urol 21:69–75. https://doi.org/10.1080/2090598X.2022.2135284
Alshuaibi M, Zugail AS, Lombion S, Beley S (2023) New protocol in the treatment of Peyronie’s disease by combining platelet-rich plasma, percutaneous needle tunneling, and penile modeling: preliminary results. Prog Urol. https://doi.org/10.1016/j.purol.2023.09.013
Chu KY, Molina ML, Ledesma B, Zucker I, Saltzman RG, Masterson TA et al (2023) A phase 2 randomized, placebo-controlled crossover trial to evaluate safety and efficacy of platelet-rich plasma injections for Peyronie’s disease: clinical trial update. Eur Urol Focus 9:11–13. https://doi.org/10.1016/j.euf.2022.08.017
Stridh A, Pontén M, Arver S, Kirsch I, Abé C, Jensen KB (2020) Placebo responses among men with erectile dysfunction enrolled in phosphodiesterase 5 inhibitor trials. JAMA Netw Open 3:e201423. https://doi.org/10.1001/jamanetworkopen.2020.1423
Gelbard M, Goldstein I, Hellstrom WJG, McMahon CG, Smith T, Tursi J et al (2013) Clinical efficacy, safety and tolerability of collagenase clostridium histolyticum for the treatment of Peyronie disease in 2 large double-blind, randomized, placebo controlled phase 3 studies. J Urol 190:199–207. https://doi.org/10.1016/j.juro.2013.01.087
Mulhall JP, Schiff J, Guhring P (2006) An analysis of the natural history of Peyronie’s disease. J Urol 175:2115–2118. https://doi.org/10.1016/S0022-5347(06)00270-9
Rosen RC, Allen KR, Ni X, Araujo AB (2011) Minimal clinically important differences in the erectile function domain of the international index of erectile function scale. Eur Urol 60:1010–1016. https://doi.org/10.1016/j.eururo.2011.07.053
De Araujo AC, Da Silva FG, Salvi F, Awad MC, Da Silva EA, Damião R (2009) The management of erectile dysfunction with placebo only: does it work? J Sex Med 6:3440–3448. https://doi.org/10.1111/j.1743-6109.2009.01496.x
Montorsi F, McDermott TED, Morgan R, Olsson A, Schultz A, Kirkeby HJ et al (1999) Efficacy and safety of fixed-dose oral sildenafil in the treatment of erectile dysfunction of various etiologies. Urology 53:1011–1018. https://doi.org/10.1016/S0090-4295(98)00643-8
Khodamoradi K, Dullea A, Golan R, Molina M, Arora H, Masterson TA et al (2022) Platelet rich plasma (PRP) growth factor concentration varies in men with erectile dysfunction. J Sex Med 19:1488–1493. https://doi.org/10.1016/j.jsxm.2022.06.003
Dhurat R, Sukesh M (2014) Principles and methods of preparation of platelet-rich plasma: a review and author′s perspective. J Cutan Aesthet Surg 7:189. https://doi.org/10.4103/0974-2077.150734
Bader R, Ibrahim JN, Moussa M, Mourad A, Azoury J, Azoury J et al (2020) In vitro effect of autologous platelet-rich plasma on H2O2 -induced oxidative stress in human spermatozoa. Andrology 8:191–200. https://doi.org/10.1111/andr.12648
Salem M, Feizollahi N, Jabari A, Golmohammadi MG, Shirinsokhan A, Ghanami Gashti N et al (2023) Differentiation of human spermatogonial stem cells using a human decellularized testicular scaffold supplemented by platelet-rich plasma. Artif Organs 47:840–853. https://doi.org/10.1111/aor.14505
Yan B, Zhang Y, Tian S, Hu R, Wu B (2021) Effect of autologous platelet-rich plasma on human sperm quality during cryopreservation. Cryobiology 98:12–16. https://doi.org/10.1016/j.cryobiol.2021.01.009
Nabavinia MS, Yari A, Ghasemi-Esmailabad S, Gholoobi A, Gholizadeh L, Nabi A et al (2023) Improvement of human sperm properties with platelet-rich plasma as a cryoprotectant supplement. Cell Tissue Bank 24:307–315. https://doi.org/10.1007/s10561-022-10032-6
Funding
Open access funding provided by Copenhagen University. None.
Author information
Authors and Affiliations
Contributions
MG Asmundo: Protocol/project development, Data collection and management, Data analysis, Manuscript writing/editing. E Durukan: Protocol/project development, Data collection and management, Data analysis, Manuscript writing/editing. E Von Rohden: Data collection and management, Data analysis, Manuscript writing/editing. SA Thy: Data collection and management, Data analysis, Manuscript writing/editing. CFS Jensen: Protocol/project development, Data collection, Manuscript writing/editing. M Fode: Protocol/project development, Data analysis, Manuscript writing/editing.
Corresponding author
Ethics declarations
Conflict of interest
Mikkel Fode is a speaker for Boston Scientific.
Human or animal participants
This systematic review does not involve any human participants or animals. Therefore, ethical approval or animal care guidelines were not required for this study.
Informed consent
Informed consent is not applicable to this narrative review manuscript as it does not involve human participants.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Asmundo, M.G., Durukan, E., von Rohden, E. et al. Platelet-rich plasma therapy in erectile dysfunction and Peyronie’s disease: a systematic review of the literature. World J Urol 42, 359 (2024). https://doi.org/10.1007/s00345-024-05065-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00345-024-05065-3