Abstract
Purpose
To investigate the feasibility, safety, and oncological outcomes of Radical Prostatectomy (RP; either Robot-Assisted [RARP] or Open RP [ORP]) in oligometastatic prostate cancer (omPCa). Additionally, we assessed whether there was an added benefit of metastasis-directed therapy (MDT) in these patients in the adjuvant setting.
Methods
Overall, 68 patients with omPCa (≤ 5 skeletal lesions at conventional imaging) treated with RP and pelvic lymph node dissection between 2006 and 2022 were included. Additional therapies (androgen deprivation therapy [ADT] and MDT) were administered according to the treating physicians’ judgment. MDT was defined as metastasis surgery/radiotherapy within 6 months of RP. We assessed Clinical Progression (CP), Biochemical Recurrence (BCR), post-operative complications and overall mortality (OM) of RP and the impact of adjuvant MDT + ADT versus RP + ADT alone.
Results
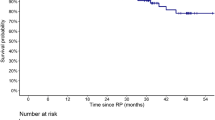
Median follow-up was 73 months (IQR 62–89). RARP reduced the risk of severe complications after adjusting for age and CCI (OR 0.15; p = 0.02). After RP, 68% patients were continent. Median 90-days PSA after RP was 0.12 ng/dL. CP and OM-free survival at 7 years were 50% and 79%, respectively. The 7-years OM-free survival rates were 93 vs. 75% for men treated with vs. without MDT (p = 0.04). At regression analyses, MDT after surgery was associated with a 70% decreased mortality rate (HR 0.27, p = 0.04).
Conclusions
RP appeared to represent a safe and feasible option in omPCa. RARP reduced the risk of severe complications. Integrating MDT with surgery in the context of a multimodal treatment might improve survival in selected omPCa patients.



Similar content being viewed by others
Data availability
The data that support the findings of this study are available on request from the corresponding author, GG.
References
Cornford P, Bellmunt J, Bolla M, Briers E, De Santis M, Gross T et al (2017) EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part II: treatment of relapsing, metastatic, and castration-resistant prostate cancer. Eur Urol 71(4):630–642
Hellman S, Weichselbaum RR. Oligometastases. J Clin Oncol 13(1):8–10. https://doi.org/10.1200/JCO.1995.13.1.8
Evangelista L, Briganti A, Fanti S, Joniau S, Reske S, Schiavina R et al (2016) New clinical indications for (18)F/(11)C-choline, new tracers for positron emission tomography and a promising hybrid device for prostate cancer staging: a systematic review of the literature. Eur Urol 70(1):161–175
Haffner MC, Mosbruger T, Esopi DM, Fedor H, Heaphy CM, Walker DA et al (2013) Tracking the clonal origin of lethal prostate cancer. J Clin Invest 123(11):4918–4922
Boevé LMS, Hulshof MCCM, Vis AN, Zwinderman AH, Twisk JWR, Witjes WPJ et al (2019) Effect on survival of androgen deprivation therapy alone compared to androgen deprivation therapy combined with concurrent radiation therapy to the prostate in patients with primary bone metastatic prostate cancer in a prospective randomised clinical trial: data from the HORRAD trial. Eur Urol 75(3):410–418
Parker CC, James ND, Brawley CD, Clarke NW, Hoyle AP, Ali A et al (2018) Radiotherapy to the primary tumour for newly diagnosed, metastatic prostate cancer (STAMPEDE): a randomised controlled phase 3 trial. The Lancet 392(10162):2353–2366
Heidenreich A, Varga Z, Knobloch RVON (2002) Extended pelvic lymphadenectomy in patients undergoing radical prostatectomy: high incidence of lymph node metastasis. J Urol 167:1681–1686
Sooriakumaran P, Karnes J, Stief C, Copsey B, Montorsi F, Hammerer P et al (2016) A multi-institutional analysis of perioperative outcomes in 106 men who underwent radical prostatectomy for distant metastatic prostate cancer at presentation. Eur Urol 69(5):788–794
Jang WS, Kim MS, Jeong WS, Chang KD, Cho KS, Ham WS et al (2018) Does robot-assisted radical prostatectomy benefit patients with prostate cancer and bone oligometastases? BJU Int 121(2):225–231
Rogowski P, Trapp C, von Bestenbostel R, Schmidt-Hegemann NS, Shi R, Ilhan H et al (2021) Outcomes of metastasis-directed therapy of bone oligometastatic prostate cancer. Radiat Oncol 16(1):125
Connor MJ, Shah TT, Horan G, Bevan CL, Winkler M, Ahmed HU (2020) Cytoreductive treatment strategies for de novo metastatic prostate cancer. Nat Rev Clin Oncol 17(3):168–182. https://pubmed.ncbi.nlm.nih.gov/31712648/
Abdollah F, Suardi N, Cozzarini C, Gallina A, Capitanio U, Bianchi M et al (2012) Selecting the optimal candidate for adjuvant radiotherapy after radical prostatectomy for prostate cancer: a long-term survival analysis. Eur Urol 63(6):998–1008. https://europepmc.org/article/med/23122664
Singh D, Yi WS, Brasacchio RA, Muhs AG, Smudzin T, Williams JP et al (2004) Is there a favorable subset of patients with prostate cancer who develop oligometastases? Int J Radiat Oncol Biol Phys 58(1):3–10
Gandaglia G, Fossati N, Stabile A (2017) Radical prostatectomy in men with oligometastatic prostate cancer: results of a single-institution series with long-term follow-up. Eur Urol 72(2):289–292
Imber BS, Varghese M, Goldman DA, Zhang Z, Gewanter R, Marciscano AE, et al (2020) Clinical outcomes of combined prostate- and metastasis-directed radiation therapy for the treatment of de novo oligometastatic prostate cancer. Adv Radiat Oncol 5(6):1213–1224. http://www.advancesradonc.org/article/S2452109420301676/fulltext
Gandaglia G, De Lorenzis E, Novara G, Fossati N, De Groote R, Dovey Z et al (2017) Robot-assisted radical prostatectomy and extended pelvic lymph node dissection in patients with locally-advanced prostate cancer. Eur Urol 71(2):249–256
Wagaskar VG, Barthe F, Martini A, Sooriakumaran P, Tewari A (2022) Oligometastatic prostate cancer: a new horizon for robotic radical prostatectomy. Mini-invasive Surg 8(6):14
Chang P, Wagner AA, Regan MM, Smith JA, Saigal CS, Litwin MS et al (2022) Prospective multicenter comparison of open and robotic radical prostatectomy: the PROST-QA/RP2 consortium. J Urol 207(1):127–136
Hussain M, Tangen CM, Higano C, Schelhammer PF, Faulkner J, Crawford ED et al (2006) Absolute prostate-specific antigen value after androgen deprivation is a strong independent predictor of survival in new metastatic prostate cancer: data from Southwest Oncology Group Trial 9346 (INT-0162). J Clin Oncol 24(24):3984–3990
O’Shaughnessy MJ, McBride SM, Vargas HA, Touijer KA, Morris MJ, Danila DC et al (2017) A pilot study of a multimodal treatment paradigm to accelerate drug evaluations in early-stage metastatic prostate cancer. Urology 1(102):164
Ost P, Reynders D, Decaestecker K, Fonteyne V, Lumen N, DeBruycker A et al (2018) Surveillance or metastasis-directed therapy for oligometastatic prostate cancer recurrence: a prospective, randomized, multicenter phase II trial. J Clin Oncol 36(5):446–453
Parker CC, James ND, Brawley CD, Clarke NW, Ali A, Amos CL et al (2022) Radiotherapy to the prostate for men with metastatic prostate cancer in the UK and Switzerland: long-term results from the STAMPEDE randomised controlled trial. PLoS Med 19(6):e1003998. https://doi.org/10.1371/journal.pmed.1003998
Ali A, Hoyle A, Haran ÁM, Brawley CD, Cook A, Amos C et al (2021) Association of bone metastatic burden with survival benefit from prostate radiotherapy in patients with newly diagnosed metastatic prostate cancer: a secondary analysis of a randomized clinical trial. JAMA Oncol 7(4):555–563. https://jamanetwork.com/journals/jamaoncology/fullarticle/2776418
Gundem G, Van Loo P, Kremeyer B, Alexandrov LB, Tubio JMC, Papaemmanuil E et al (2015) The evolutionary history of lethal metastatic prostate cancer. Nature 520(7547):353–357
Deek MP, Van der Eecken K, Phillips R, Parikh NR, Isaacsson Velho P, Lotan TL et al (2021) The mutational landscape of metastatic castration-sensitive prostate cancer: the spectrum theory revisited. Eur Urol 80(5):632–640
Conteduca V, Hess J, Yamada Y, Ku SY, Beltran H (2021) Epigenetics in prostate cancer: clinical implications. Transl Androl Urol 10(7):3104
Dhondt B, De Bleser E, Claeys T, Buelens S, Lumen N, Vandesompele J et al (2019) Discovery and validation of a serum microRNA signature to characterize oligo- and polymetastatic prostate cancer: not ready for prime time. World J Urol 37(12):2557–2564
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
AP: project development, data collection, manuscript writing/editing. GG: project development, manuscript editing. MA: data collection. GF: data collection. EM: data analysis. AS: project development. FP: data analysis. DR: data collection. RL: data collection. SS: data collection. VC: data analysis. GOC: data collection. FB: data collection. FM: project development, manuscript editing. AB: project development, manuscript editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors of this article disclose that they have no potential conflicts of interest.
Human participants
This research present in this manuscript involving human participants has been conducted in compliance with all relevant ethical guidelines and regulations.
Informed consent
Informed consent was obtained from all human participants included in this research.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pellegrino, A., Gandaglia, G., de Angelis, M. et al. Oncological and perioperative outcomes of surgery with or without metastasis-directed therapy as part of a multimodal treatment in men with de-novo oligometastatic prostate cancer. World J Urol 41, 2069–2076 (2023). https://doi.org/10.1007/s00345-023-04460-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-023-04460-6