Abstract
Purpose
MAUDE database houses medical device reports of suspected device-related complications received by Food and Drug Administration. In the present study we aim to evaluate the MAUDE database for reported complications of MIST procedures.
Methods
The database was queried using keywords: rezum, urolift, prostate embolization (PAE), transurethral needle ablation (TUNA), transurethral microwave therapy (TUMT), prostate stent and Temporarily Implanted Nitinol Device (iTIND) on 10/1/22 to extract information regarding device problems and procedure-related complications. Gupta classification system was used to stratify complications. Statistical analysis was performed to compare frequency of complications among MIST procedures.
Results
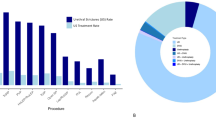
We found a total of 692 reports (Rezum-358, urolift-226, PAE-53, TUNA-31, TUMT-19, prostatic stent-4, and iTIND-1). Most complications related to device or users were minor (level 1 and 2) and there was no significant difference among various MIST procedures. The screen/system error was responsible for 93% and 83% aborted cases in Rezum and TUNA, respectively, and PAE showed 40% of device component detachment/fracture. Overall Urolift and TUMT were associated with statistically significant higher incidence of major (level 3 and 4) complications (23% and 21%, respectively) as compared with Rezum (7%). Most major complications needing hospitalization after Urolift included hematoma and hematuria with clots and those after Rezum included urinary tract infection and sepsis. Thirteen deaths were reported, mostly due to cardiovascular events, which were classified as not associated with the proposed treatment.
Conclusion
MIST for BPH can occasionally cause significant morbidity. Our data should assist urologists and patients in shared decision-making process.

Similar content being viewed by others
Data Availability
The data would be made available upon request by corresponding author.
Abbreviations
- AE:
-
Adverse event
- AUA:
-
American Urological Association
- BPH:
-
Benign prostatic hyperplasia
- EAU:
-
European Association of Urology
- FDA:
-
Food and Drug Administration
- iTIND:
-
Temporarily Implanted Nitinol Device
- LUTS:
-
Lower urinary tract symptoms
- MAUDE:
-
Manufacturer and User Facility Device Experience
- MIST:
-
Minimally invasive surgical therapies
- OML:
-
Obstructive middle lobe
- PAE:
-
Prostate artery embolization
- PUL:
-
Prostatic urethral lift
- RCT:
-
Randomized clinical trial
- TUMT:
-
Transurethral microwave therapy
- TUNA:
-
Transurethral needle ablation
- TURP:
-
Transurethral resection of the prostate
- UTI:
-
Urinary tract infection
References
Berry SJ, Coffey DS, Walsh PC, Ewing LL (1984) The development of human benign prostatic hyperplasia with age. J Urol. https://doi.org/10.1016/S0022-5347(17)49698-4
Lerner LB, McVary KT, Barry MJ, Bixler BR, Dahm P, Das AK, Gandhi MC, Kaplan SA, Kohler TS, Martin L, Parsons JK, Roehrborn CG, Stoffel JT, Welliver C, Wilt TJ (2021) Management of lower urinary tract symptoms attributed to benign prostatic hyperplasia: AUA GUIDELINE PART I-initial work-up and medical management. J Urol. https://doi.org/10.1097/JU.0000000000002183
Gravas S, Cornu JN, Gacci M, Gratzke C, Herrmann TRW, Mamoulakis C, Rieken M, Speakman MJ, Tikkinen KAO, Guidelines Associates: Karavitakis M, Kyriazis I, Malde S, Sakalis V, Guidelines Office: Schouten N, Smith EJ (2022) EAU guidelines on management of non-neurogenic male lower urinary tract symptoms (LUTS), incl. benign prostatic obstruction (BPO). In: Presented at the EAU annual congress Amsterdam 2022
Checcucci E et al (2021) New ultra-minimally invasive surgical treatment for benign prostatic hyperplasia: a systematic review and analysis of comparative outcomes. Eur Urol Open Sci. https://doi.org/10.1016/j.euros.2021.08.009
Manfredi C et al (2022) Emerging minimally invasive transurethral treatments for benign prostatic hyperplasia: a systematic review with meta-analysis of functional outcomes and description of complications. Minerva Urol Nephrol. https://doi.org/10.23736/S2724-6051.21.04530-4
Bouhadana D, Nguyen D-D, Zorn KC, Elterman DS, Bhojani N (2020) Patient perspectives on benign prostatic hyperplasia surgery: a focus on sexual health. J Sex Med. https://doi.org/10.1016/j.jsxm.2020.07.006
Gurtcheff SE (2008) Introduction to the MAUDE Database. Clin Obstet Gynecol. https://doi.org/10.1097/GRF.0b013e318161e657
MAUDE—Manufacturer and User Facility Device Experience. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfmaude/search.cfm
Kaplan-Marans E, Martinez M, Wood A, Cochran J, Dubowitch E, Schulman A (2022) Aquablation, prostatic urethral lift, and transurethral water vapor therapy: a comparison of device-related adverse events in a national registry. J Endourol. https://doi.org/10.1089/end.2021.0455
Patel NH et al (2019) Device malfunctions and complications associated with benign prostatic hyperplasia surgery: review of the manufacturer and user facility device experience database. J Endourol. https://doi.org/10.1089/end.2019.0067
Weiss JK, Santucci NM, Sajadi KP, Chouhan JD (2021) Post-surgical complications after bladder outlet reducing surgery: an analysis of the FDA manufacturer and user facility device experience (MAUDE) database. Urology. https://doi.org/10.1016/j.urology.2021.04.030
Gupta P et al (2017) Development of a classification scheme for examining adverse events associated with medical devices, specifically the DaVinci surgical system as reported in the FDA MAUDE database. J Endourol. https://doi.org/10.1089/end.2016.0396
McVary KT et al (2016) Minimally invasive prostate convective water vapor energy ablation: a multicenter, randomized, controlled study for the treatment of lower urinary tract symptoms secondary to benign prostatic hyperplasia. J Urol. https://doi.org/10.1016/j.juro.2015.10.181
McVary KT et al (2016) Erectile and ejaculatory function preserved with convective water vapor energy treatment of lower urinary tract symptoms secondary to benign prostatic hyperplasia: randomized controlled study. J Sex Med. https://doi.org/10.1016/j.jsxm.2016.03.372
McVary KT, Rogers T, Roehrborn CG (2019) Rezūm water vapor thermal therapy for lower urinary tract symptoms associated with benign prostatic hyperplasia: 4-year results from randomized controlled study. Urology. https://doi.org/10.1016/j.urology.2018.12.041
Woo HH, Chin PT, McNicholas TA, Gill HS, Plante MK, Bruskewitz RC, Roehrborn CG (2011) Safety and feasibility of the prostatic urethral lift: a novel, minimally invasive treatment for lower urinary tract symptoms (LUTS) secondary to benign prostatic hyperplasia (BPH). BJU Int. https://doi.org/10.1111/j.1464-410X.2011.10342.x
Franco JVA et al (2022) Minimally invasive treatments for benign prostatic hyperplasia: a Cochrane network meta-analysis. BJU Int. https://doi.org/10.1111/bju.15653
Ng BHS, Chung E (2021) A state-of-art review on the preservation of sexual function among various minimally invasive surgical treatments for benign prostatic hyperplasia: Impact on erectile and ejaculatory domains. Investig Clin Urol. https://doi.org/10.4111/icu.20200392
Roehmholdt MJ, Bentley DF (2022) Large pelvic hematoma after UroLift® procedure for treatment of BPH with median lobe. Case Rep Urol. https://doi.org/10.1155/2022/7065865
Rukstalis D et al (2019) Prostatic Urethral Lift (PUL) for obstructive median lobes: 12 month results of the MedLift Study. Prostate Cancer Prostat Dis. https://doi.org/10.1038/s41391-018-0118-x
Carnevale FC et al (2020) Prostatic artery embolization for the treatment of lower urinary tract symptoms due to benign prostatic hyperplasia: 10 years’ experience. Radiology. https://doi.org/10.1148/radiol.2020191249
Ray AF et al (2018) Efficacy and safety of prostate artery embolization for benign prostatic hyperplasia: an observational study and propensity-matched comparison with transurethral resection of the prostate (the UK-ROPE study). BJU Int. https://doi.org/10.1111/bju.14249
Pisco JM et al (2016) Medium- and long-term outcome of prostate artery embolization for patients with benign prostatic hyperplasia: results in 630 patients. J Vasc Interv Radiol. https://doi.org/10.1016/j.jvir.2016.04.001
Svarc P, Taudorf M, Nielsen MB, Stroomberg HV, Røder MA, Lönn L (2020) Postembolization syndrome after prostatic artery embolization: a systematic review. Diagnostics. https://doi.org/10.3390/diagnostics10090659
Pilan BF, de Assis AM, Moreira AM, de Rodrigues VCP, Carnevale FC (2022) Protection of nontarget structures in prostatic artery embolization. Radiol Bras. https://doi.org/10.1590/0100-3984.2021.0021
Bouza C, López T, Magro A, Navalpotro L, Amate JM (2006) Systematic review and meta-analysis of transurethral needle ablation in symptomatic benign prostatic hyperplasia. BMC Urol. https://doi.org/10.1186/1471-24906-14
Albala DM et al (2002) Office-based transurethral microwave thermotherapy using the TherMatrx TMx-2000. J Endourol. https://doi.org/10.1089/089277902753483745
Masood S, Djaladat H, Kouriefs C, Keen M, Palmer JH (2004) The 12-year outcome analysis of an endourethral wallstent for treating benign prostatic hyperplasia. BJU Int. https://doi.org/10.1111/j.1464-410X.2004.05155.x
Chughtai B et al (2021) The iTind temporarily implanted nitinol device for the treatment of lower urinary tract symptoms secondary to benign prostatic hyperplasia: a multicenter, randomized, controlled trial. Urology. https://doi.org/10.1016/j.urology.2020.12.022
Porpiglia F et al (2019) Second-generation of temporary implantable nitinol device for the relief of lower urinary tract symptoms due to benign prostatic hyperplasia: results of a prospective, multicentre study at 1 year of follow-up. BJU Int. https://doi.org/10.1111/bju.14608
Funding
None.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. All authors read and approved the final manuscript. JGP contributed to data collection and manuscript writing/editing. MCSA contributed to manuscript writing/editing. RBB contributed to data analysis and manuscript writing/editing. A Bhatia contributed to manuscript writing/editing. SB contributed to manuscript writing/editing. RS contributed to manuscript writing/editing. RM contributed to manuscript writing/editing. HNS contributed to project development, data management, and manuscript writing/editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interests for this study.
Ethics approval
It was not necessary for this study.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Porto, J.G., Arbelaez, M.C.S., Blachman-Braun, R. et al. Complications associated with minimally invasive surgical therapies (MIST) for surgical management of benign prostatic hyperplasia: a Manufacturer and User Facility Device Experience (MAUDE) database review. World J Urol 41, 1975–1982 (2023). https://doi.org/10.1007/s00345-023-04440-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-023-04440-w