Abstract
Purpose
To summarize contemporary and emerging strategies for the diagnosis and management of metastatic hormone sensitive prostate cancer (mHSPC), focusing on diagnostic testing and therapeutics.
Methods
Literature review using PUBMED-Medline databases as well as clinicaltrials.gov to include reported or ongoing clinical trials on treatment for mHSPC. We prioritized the findings from phase III randomized clinical trials, systematic reviews, meta-analyses and clinical practice guidelines.
Results
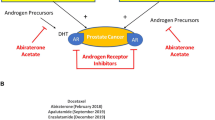
There have been significant changes to the diagnosis and staging evaluation of mHSPC with the integration of increasingly accurate positron emission tomography (PET) imaging tracers that exceed the performance of conventional computerized tomography (CT) and bone scan. Germline multigene testing is recommended for the evaluation of patients newly diagnosed with mHSPC given the prevalence of actionable alterations that may create candidacy for specific therapies. Although androgen deprivation therapy (ADT) remains the backbone of treatment for mHSPC, approaches to first-line treatment include the integration of multiple agents including androgen receptor synthesis inhibitors (ARSI; abiraterone) Androgen Receptor antagonists (enzalutamide, darolutamide, apalautamide), and docetaxel chemotherapy. The combination of ADT, ARSI, and docetaxel chemotherapy has recently been evaluated in a randomized trial and was associated with significantly improved overall survival including in patients with a high burden of disease. The role of local treatment to the prostate with radiation has been evaluated in randomized trials with additional studies underway evaluating the role of cytoreductive radical prostatectomy.
Conclusion
The staging and initial management of patients with mHSPC has undergone significant advances in the last decade with advancements in the diagnosis, treatment and sequencing of therapies.
Similar content being viewed by others
References
Rawla P (2019) Epidemiology of prostate cancer. World J Oncol 10:63–89. https://doi.org/10.14740/wjon1191
Siegel RL, Miller KD, Fuchs HE, Jemal A (2022) Cancer statistics, 2002. CA A Cancer J Clin. https://doi.org/10.3322/caac.21708
Carlsson S et al (2012) Prostate cancer screening: facts, statistics, and interpretation in response to the US preventive services task force review. J Clin Oncol 30:2581
Patel NH et al (2018) Prostate cancer screening trends after united states preventative services task force guidelines in an underserved population. Health Equity 2:55–61. https://doi.org/10.1089/heq.2018.0004
Leapman MS et al (2022) Changes in prostate-specific antigen testing relative to the revised US preventive services task force recommendation on prostate cancer screening. JAMA Oncol 8:41–47. https://doi.org/10.1001/jamaoncol.2021.5143
Ahlering T et al (2019) Unintended consequences of decreased PSA-based prostate cancer screening. World J Urol 37:489–496. https://doi.org/10.1007/s00345-018-2407-3
Dall’Era MA, deVere-White R, Rodriguez D, Cress R (2019) Changing incidence of metastatic prostate cancer by race and age, 1988–2015. Eur Urol Focus 5:1014–1021. https://doi.org/10.1016/j.euf.2018.04.016
Desai MM et al (2022) Trends in incidence of metastatic prostate cancer in the US. JAMA Netw Open 5:e222246. https://doi.org/10.1001/jamanetworkopen.2022.2246
Wade CA, Kyprianou N (2018) Profiling prostate cancer therapeutic resistance. Int J Mol Sci. https://doi.org/10.3390/ijms19030904
Khoshkar Y et al (2022) Mortality in men with castration-resistant prostate cancer-A long-term follow-up of a population-based real-world cohort. BJUI Compass 3:173–183. https://doi.org/10.1002/bco2.116
Das CJ, Razik A, Sharma S (2018) Positron emission tomography in prostate cancer: an update on state of the art. Indian J Urol IJU J Urol Soc India 34:172–179. https://doi.org/10.4103/iju.IJU_320_17
Giri VN et al (2020) Implementation of germline testing for prostate cancer: Philadelphia prostate cancer consensus Conference 2019. J Clin Oncol 38:2798–2811. https://doi.org/10.1200/jco.20.00046
Lowrance WT et al (2021) Advanced prostate cancer: AUA/ASTRO/SUO guideline PART I. J Urol 205:14–21. https://doi.org/10.1097/ju.0000000000001375
Overman D (2022) Lantheus updates NCCN guidelines for PSMA PET imaging for prostate cancer. AXIS imaging news
Ljungberg B et al (2020) EAU guidelines. Edn presented at the EAU annual congress Amsterdam. Eur Urol 67:913–924
Wenzel M et al (2021) Overall survival after systemic treatment in high-volume versus low-volume metastatic hormone-sensitive prostate cancer: systematic review and network meta-analysis. Eur Urol Focus. https://doi.org/10.1016/j.euf.2021.04.003
Sweeney CJ et al (2015) Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N Engl J Med 373:737–746. https://doi.org/10.1056/NEJMoa1503747
Jia JB, Houshyar R, Verma S, Uchio E, Lall C (2016) Prostate cancer on computed tomography: A direct comparison with multi-parametric magnetic resonance imaging and tissue pathology. Eur J Radiol 85:261–267. https://doi.org/10.1016/j.ejrad.2015.10.013
Mason BR et al (2019) Current status of MRI and PET in the NCCN guidelines for prostate cancer. J Natl Compr Canc Netw 17:506–513. https://doi.org/10.6004/jnccn.2019.7306
Lecouvet FE et al (2012) Can whole-body magnetic resonance imaging with diffusion-weighted imaging replace Tc 99m bone scanning and computed tomography for single-step detection of metastases in patients with high-risk prostate cancer? Eur Urol 62:68–75. https://doi.org/10.1016/j.eururo.2012.02.020
Soloway MS et al (1988) Stratification of patients with metastatic prostate cancer based on extent of disease on initial bone scan. Cancer 61:195–202. https://doi.org/10.1002/1097-0142(19880101)61:1%3c195::aid-cncr2820610133%3e3.0.co;2-y
Conteduca V et al (2021) Flare phenomenon in prostate cancer: recent evidence on new drugs and next generation imaging. Ther Adv Med Oncol 13:1758835920987654. https://doi.org/10.1177/1758835920987654
Fizazi K et al (2019) Abiraterone acetate plus prednisone in patients with newly diagnosed high-risk metastatic castration-sensitive prostate cancer (LATITUDE): final overall survival analysis of a randomised, double-blind, phase 3 trial. Lancet Oncol 20:686–700. https://doi.org/10.1016/s1470-2045(19)30082-8
Niaz MJ et al (2021) Review of commonly used prostate specific PET tracers used in prostate cancer imaging in current clinical practice. Clin Imag 79:278–288. https://doi.org/10.1016/j.clinimag.2021.06.006
Patel DN, Karsh LI, Daskivich TJ (2021) Next-generation imaging in localized high-risk prostate cancer. Prostate Cancer Prostatic Dis 24:585–586
Armstrong JM et al (2020) (18)F-fluciclovine PET CT detection of biochemical recurrent prostate cancer at specific PSA thresholds after definitive treatment. Urol Oncol 38(636):e631-636.e636. https://doi.org/10.1016/j.urolonc.2020.03.021
Wu SY et al (2019) Impact of staging (68)Ga-PSMA-11 PET scans on radiation treatment plansin patients with prostate cancer. Urology 125:154–162. https://doi.org/10.1016/j.urology.2018.09.038
Zacho HD et al (2020) Added value of (68)Ga-PSMA PET/CT for the detection of bone metastases in patients with newly diagnosed prostate cancer and a previous (99m)Tc bone scintigraphy. EJNMMI Res 10:31. https://doi.org/10.1186/s13550-020-00618-0
Hofman MS et al (2020) Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): a prospective, randomised, multicentre study. Lancet (London, England) 395:1208–1216. https://doi.org/10.1016/s0140-6736(20)30314-7
Martorana G et al (2006) 11C-choline positron emission tomography/computerized tomography for tumor localization of primary prostate cancer in comparison with 12-core biopsy. J Urol 176:954–960. https://doi.org/10.1016/j.juro.2006.04.015
Sheikhbahaei S et al (2019) (18)F-NaF-PET/CT for the detection of bone metastasis in prostate cancer: a meta-analysis of diagnostic accuracy studies. Ann Nucl Med 33:351–361. https://doi.org/10.1007/s12149-019-01343-y
Perera M et al (2016) Sensitivity, specificity, and predictors of positive (68)Ga-prostate-specific membrane antigen positron emission tomography in advanced prostate cancer: a systematic review and meta-analysis. Eur Urol 70:926–937. https://doi.org/10.1016/j.eururo.2016.06.021
Pan KH et al (2020) Evaluation of 18F-DCFPyL PSMA PET/CT for prostate cancer: a meta-analysis. Front Oncol 10:597422. https://doi.org/10.3389/fonc.2020.597422
Kim SJ, Lee SW (2019) The role of (18)F-fluciclovine PET in the management of prostate cancer: a systematic review and meta-analysis. Clin Radiol 74:886–892. https://doi.org/10.1016/j.crad.2019.06.022
Hussain M et al (2022) evolving role of prostate-specific membrane antigen-positron emission tomography in metastatic hormone-sensitive prostate cancer: more questions than answers? J Clin Oncol 40:3011–3014. https://doi.org/10.1200/jco.22.00208
Jadvar H et al (2022) Appropriate use criteria for prostate-specific membrane antigen PET imaging. J Nucl Med 63:59–68. https://doi.org/10.2967/jnumed.121.263262
Nakanishi K et al (2022) Whole-body MRI: detecting bone metastases from prostate cancer. Jpn J Radiol 40:229–244. https://doi.org/10.1007/s11604-021-01205-6
Shen G, Deng H, Hu S, Jia Z (2014) Comparison of choline-PET/CT, MRI, SPECT, and bone scintigraphy in the diagnosis of bone metastases in patients with prostate cancer: a meta-analysis. Skeletal Radiol 43:1503–1513. https://doi.org/10.1007/s00256-014-1903-9
Padhani AR et al (2017) METastasis reporting and data system for prostate cancer: practical guidelines for acquisition, interpretation, and reporting of whole-body magnetic resonance imaging-based evaluations of multiorgan involvement in advanced prostate cancer. Eur Urol 71:81–92. https://doi.org/10.1016/j.eururo.2016.05.033
Pricolo P et al (2020) Whole-body magnetic resonance imaging (WB-MRI) reporting with the METastasis Reporting and Data System for Prostate Cancer (MET-RADS-P): inter-observer agreement between readers of different expertise levels. Cancer Imaging 20:77. https://doi.org/10.1186/s40644-020-00350-x
Biloglav Z et al (2020) The analysis of waiting time and utilization of computed tomography and magnetic resonance imaging in Croatia: a nationwide survey. Croat Med J 61:538–546. https://doi.org/10.3325/cmj.2020.61.538
Giri VN et al (2020) Implementation of germline testing for prostate cancer: Philadelphia prostate cancer consensus conference 2019. J Clin Oncol 38:2798–2811. https://doi.org/10.1200/jco.20.00046
Cheng HH, Sokolova AO, Schaeffer EM, Small EJ, Higano CS (2019) Germline and somatic mutations in prostate cancer for the clinician. J Natl Compr Canc Netw 17:515–521. https://doi.org/10.6004/jnccn.2019.7307
Beebe-Dimmer JL, Kapron AL, Fraser AM, Smith KR, Cooney KA (2020) Risk of prostate cancer associated with familial and hereditary cancer syndromes. J Clin Oncol 38:1807–1813. https://doi.org/10.1200/jco.19.02808
Pritchard CC et al (2016) Inherited DNA-repair gene mutations in men with metastatic prostate cancer. N Engl J Med 375:443–453. https://doi.org/10.1056/NEJMoa1603144
Paller CJ et al (2019) Germline genetic testing in advanced prostate cancer; practices and barriers: survey results from the germline genetics working group of the prostate cancer clinical trials consortium. Clin Genitourin Cancer 17:275-282.e271. https://doi.org/10.1016/j.clgc.2019.04.013
Thorne H et al (2011) Decreased prostate cancer-specific survival of men with BRCA2 mutations from multiple breast cancer families. Cancer Prev Res (Phila) 4:1002–1010. https://doi.org/10.1158/1940-6207.Capr-10-0397
Castro E et al (2015) Effect of BRCA mutations on metastatic relapse and cause-specific survival after radical treatment for localised prostate cancer. Eur Urol 68:186–193. https://doi.org/10.1016/j.eururo.2014.10.022
Faraoni I, Graziani G (2018) Role of BRCA mutations in cancer treatment with poly(ADP-ribose) polymerase (PARP) inhibitors. Cancers (Basel). https://doi.org/10.3390/cancers10120487
Castro E, Eeles R (2012) The role of BRCA1 and BRCA2 in prostate cancer. Asian J Androl 14:409–414. https://doi.org/10.1038/aja.2011.150
Cheng HH, Pritchard CC, Boyd T, Nelson PS, Montgomery B (2016) Biallelic inactivation of BRCA2 in platinum-sensitive metastatic castration-resistant prostate cancer. Eur Urol 69:992–995. https://doi.org/10.1016/j.eururo.2015.11.022
Pomerantz MM et al (2017) The association between germline BRCA2 variants and sensitivity to platinum-based chemotherapy among men with metastatic prostate cancer. Cancer 123:3532–3539. https://doi.org/10.1002/cncr.30808
Zheng G et al (2016) Clinical mutational profiling of bone metastases of lung and colon carcinoma and malignant melanoma using next-generation sequencing. Cancer Cytopathol 124:744–753. https://doi.org/10.1002/cncy.21743
Ng SWS, Wyatt AW (2021) Building confidence in circulating tumour DNA assays for metastatic castration-resistant prostate cancer. Nat Rev Urol 18:255–256. https://doi.org/10.1038/s41585-021-00455-3
Siravegna G et al (2019) How liquid biopsies can change clinical practice in oncology. Ann Oncol 30:1580–1590. https://doi.org/10.1093/annonc/mdz227
Choudhury AD (2022) PTEN-PI3K pathway alterations in advanced prostate cancer and clinical implications. Prostate 82(Suppl 1):S60-s72. https://doi.org/10.1002/pros.24372
Kluth LA et al (2014) The hypothalamic-pituitary-gonadal axis and prostate cancer: implications for androgen deprivation therapy. World J Urol 32:669–676. https://doi.org/10.1007/s00345-013-1157-5
Dai C, Heemers H, Sharifi N (2017) Androgen signaling in prostate cancer. Cold Spring Harb Perspect Med. https://doi.org/10.1101/cshperspect.a030452
Shore ND et al (2020) Oral relugolix for androgen-deprivation therapy in advanced prostate cancer. N Engl J Med 382:2187–2196. https://doi.org/10.1056/NEJMoa2004325
Potter GA, Barrie SE, Jarman M, Rowlands MG (1995) Novel steroidal inhibitors of human cytochrome P45017 alpha (17 alpha-hydroxylase-C17,20-lyase): potential agents for the treatment of prostatic cancer. J Med Chem 38:2463–2471. https://doi.org/10.1021/jm00013a022
Danila DC et al (2010) Phase II multicenter study of abiraterone acetate plus prednisone therapy in patients with docetaxel-treated castration-resistant prostate cancer. J Clin Oncol 28:1496–1501. https://doi.org/10.1200/jco.2009.25.9259
Fizazi K et al (2012) Abiraterone acetate for treatment of metastatic castration-resistant prostate cancer: final overall survival analysis of the COU-AA-301 randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol 13:983–992. https://doi.org/10.1016/s1470-2045(12)70379-0
Ryan CJ et al (2015) Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (COU-AA-302): final overall survival analysis of a randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol 16:152–160. https://doi.org/10.1016/s1470-2045(14)71205-7
Fizazi K et al (2017) Abiraterone plus prednisone in metastatic, castration-sensitive prostate cancer. N Engl J Med 377:352–360. https://doi.org/10.1056/NEJMoa1704174
James ND et al (2017) Abiraterone for prostate cancer not previously treated with hormone therapy. N Engl J Med 377:338–351. https://doi.org/10.1056/NEJMoa1702900
James N et al (2020) 611O Abiraterone acetate plus prednisolone for hormone-naïve prostate cancer (PCa): Long-term results from metastatic (M1) patients in the STAMPEDE randomised trial (NCT00268476). Ann Oncol 31:S509
Lokeshwar SD, Klaassen Z, Saad F (2021) Treatment and trials in non-metastatic castration-resistant prostate cancer. Nat Rev Urol 18:433–442. https://doi.org/10.1038/s41585-021-00470-4
Davis ID et al (2019) Enzalutamide with standard first-line therapy in metastatic prostate cancer. N Engl J Med 381:121–131. https://doi.org/10.1056/NEJMoa1903835
Armstrong AJ et al (2019) ARCHES: a randomized, phase III study of androgen deprivation therapy with enzalutamide or placebo in men with metastatic hormone-sensitive prostate cancer. J Clin Oncol 37:2974–2986. https://doi.org/10.1200/jco.19.00799
Armstrong AJ et al (2022) Improved survival with enzalutamide in patients with metastatic hormone-sensitive prostate cancer. J Clin Oncol. https://doi.org/10.1200/jco.22.00193
Clegg NJ et al (2012) ARN-509: a novel antiandrogen for prostate cancer treatment. Cancer Res 72:1494–1503. https://doi.org/10.1158/0008-5472.Can-11-3948
Chi KN et al (2019) Apalutamide for metastatic, castration-sensitive prostate cancer. N Engl J Med 381:13–24. https://doi.org/10.1056/NEJMoa1903307
Chi KN et al (2021) Am Soc Clin Oncol
Mukhtar E, Adhami VM, Mukhtar H (2014) Targeting microtubules by natural agents for cancer therapy. Mol Cancer Ther 13:275–284. https://doi.org/10.1158/1535-7163.MCT-13-0791
Tannock IF et al (2004) Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med 351:1502–1512. https://doi.org/10.1056/NEJMoa040720
Kyriakopoulos CE et al (2018) Chemohormonal therapy in metastatic hormone-sensitive prostate cancer: long-term survival analysis of the randomized phase III E3805 CHAARTED trial. J Clin Oncol 36:1080–1087. https://doi.org/10.1200/jco.2017.75.3657
Clarke NW et al (2019) Addition of docetaxel to hormonal therapy in low- and high-burden metastatic hormone sensitive prostate cancer: long-term survival results from the STAMPEDE trial. Ann Oncol 30:1992–2003. https://doi.org/10.1093/annonc/mdz396
James ND et al (2016) Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet (London, England) 387:1163–1177. https://doi.org/10.1016/s0140-6736(15)01037-5
Gravis G et al (2013) Androgen-deprivation therapy alone or with docetaxel in non-castrate metastatic prostate cancer (GETUG-AFU 15): a randomised, open-label, phase 3 trial. Lancet Oncol 14:149–158. https://doi.org/10.1016/s1470-2045(12)70560-0
Fizazi K et al (2022) Abiraterone plus prednisone added to androgen deprivation therapy and docetaxel in de novo metastatic castration-sensitive prostate cancer (PEACE-1): a multicentre, open-label, randomised, phase 3 study with a 2 × 2 factorial design. Lancet (London, England), https://doi.org/10.1016/s0140-6736(22)00367-1
Moilanen AM et al (2015) Discovery of ODM-201, a new-generation androgen receptor inhibitor targeting resistance mechanisms to androgen signaling-directed prostate cancer therapies. Sci Rep 5:12007. https://doi.org/10.1038/srep12007
Zurth C, Sandmann S, Trummel D, Seidel D, Gieschen H (2018) Blood-brain barrier penetration of [14C] darolutamide compared with [14C] enzalutamide in rats using whole body autoradiography. Am Soc Clin Oncol
Smith MR et al (2022) Darolutamide and survival in metastatic, hormone-sensitive prostate cancer. N Engl J Med 386:1132–1142. https://doi.org/10.1056/NEJMoa2119115
Lee CE et al (2011) A comprehensive bone-health management approach for men with prostate cancer receiving androgen deprivation therapy. Curr Oncol 18:e163-172. https://doi.org/10.3747/co.v18i4.746
Yu EY et al (2015) SWOG S0925: a randomized phase ii study of androgen deprivation combined with cixutumumab versus androgen deprivation alone in patients with new metastatic hormone-sensitive prostate cancer. J Clin Oncol 33:1601–1608. https://doi.org/10.1200/jco.2014.59.4127
Parker CC et al (2018) Radiotherapy to the primary tumour for newly diagnosed, metastatic prostate cancer (STAMPEDE): a randomised controlled phase 3 trial. Lancet (London, England) 392:2353–2366. https://doi.org/10.1016/s0140-6736(18)32486-3
Boevé LMS et al (2019) Effect on survival of androgen deprivation therapy alone compared to androgen deprivation therapy combined with concurrent radiation therapy to the prostate in patients with primary bone metastatic prostate cancer in a prospective randomised clinical trial: data from the HORRAD trial. Eur Urol 75:410–418. https://doi.org/10.1016/j.eururo.2018.09.008
Wang Y et al (2020) Comparative efficacy of combined radiotherapy, systemic therapy, and androgen deprivation therapy for metastatic hormone-sensitive prostate cancer: a network meta-analysis and systematic review. Front Oncol 10:567616. https://doi.org/10.3389/fonc.2020.567616
Ranasinghe W, Chapin BF, Kim IY, Sooriakumaran P, Lawrentschuk N (2020) The cytoreductive prostatectomy in metastatic prostate cancer: what the individual trials are hoping to answer. BJU Int 125:792–800. https://doi.org/10.1111/bju.15055
Sooriakumaran P et al (2021) Feasibility and safety of radical prostatectomy for oligo-metastatic prostate cancer: the Testing Radical prostatectomy in men with prostate cancer and oligo-Metastases to the bone (TRoMbone) trial. BJU Int. https://doi.org/10.1111/bju.15669
Yuh BE et al (2019) Results of phase 1 study on cytoreductive radical prostatectomy in men with newly diagnosed metastatic prostate cancer. Prostate Int 7:102–107. https://doi.org/10.1016/j.prnil.2018.10.002
Kim IY et al (2022) Genomic analysis and long-term outcomes of a phase 1 clinical trial on cytoreductive radical prostatectomy. Prostate Int 10:75–79. https://doi.org/10.1016/j.prnil.2022.03.001
Heidenreich A et al (2018) Cytoreductive radical prostatectomy in men with prostate cancer and skeletal metastases. Eur Urol Oncol 1:46–53. https://doi.org/10.1016/j.euo.2018.03.002
Author information
Authors and Affiliations
Contributions
SD Lokeshwar: protocol/project development, data collection and management, data analysis, manuscript writing/editing. AU Choksi: data collection and management, data analysis, manuscript writing/editing. D Haltstuch: manuscript writing/ editing. SN Rahman: manuscript writing/editing. BH Press: manuscript writing/editing. J Syed: protocol/ project development, manuscript editing. ME Hurwitz: manuscript editing. IY Kim: manuscript editing. MS Leapman: protocol/project development, data collection and management, data analysis, manuscript writing/editing.
Corresponding author
Ethics declarations
Conflict of interest
Disclosure of potential conflicts of interest: SD Lokeshwar, AU Choksi, D Haltstuch, SN Rahman, BH Press, J syed, ME Hurwitz, IY Kim, MS Leapman have no potential conflict of interest.
Research involving human participants and/or animals
Not applicable to this narrative review.
Informed consent
Not applicable to this narrative review.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lokeshwar, S.D., Choksi, A.U., Haltstuch, D. et al. Personalizing approaches to the management of metastatic hormone sensitive prostate cancer: role of advanced imaging, genetics and therapeutics. World J Urol 41, 2007–2019 (2023). https://doi.org/10.1007/s00345-023-04409-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-023-04409-9