Abstract
Purpose
Adrenergic and cholinergic pathways play an important role in contraction–relaxation harmony in human bladder. Functional changes in any proteins in these pathways may result in overactive bladder. We aimed to investigate whether single gene polymorphisms affecting adrenergic and cholinergic pathways are associated with OAB syndrome.
Methods
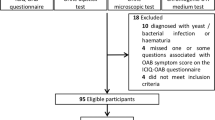
60 patients with idiopathic OAB and 60 healthy controls were included in the study. A validated OAB-V8 questionnaire was given to all patients. Polymorphisms of ADRB3, ROCK2, and GEF gene were detected by PCR from whole blood samples. Genotypic structures of patients and controls were compared. The relationship between genotypic structures and OAB symptom scores were investigated.
Results
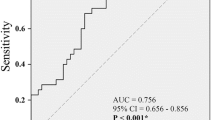
We found no significant difference in the genotype and allele frequencies between the patients and controls for all three SNP. While there was no relationship between ADRB3 and GEF gene polymorphisms and OAB scores in OAB patients, the OAB score in heterozygous polymorphic individuals was significantly higher than in homozygous polymorphic individuals in the ROCK2 gene (p = 0.039).
Conclusion
The polymorphisms of the ADRB3, ROCK2, and GEF genes were present in both OAB group and healthy controls, but were not associated with OAB syndrome.
Similar content being viewed by others
References
Abrams P, Cardozo L, Fall M et al (2003) The standardisation of terminology of lower urinary tract function: report from the Standardisation Sub-committee of the International Continence Society. Neurol Urodyn Urol 61(1):37–49
Jenks JC (2016) Overactive bladder in women. Nurs Stand 31(9):52–63
Yoshimura N, de Groat WC (1997) Neural control of the lower urinary tract. Int J Urol 2:111–125
Yamaguchi O (2002) ß3-adrenoceptors in human detrusor muscle. Urology 59(5 suppl 1):25–29
Emorine LJ, Marullo S, Briden-sutren MM et al (1989) Molecular characterization of the human b3-adrenergic receptor. Science 245(4922):1118–1121
Clement K, Vaisse C, Manning BS et al (1995) Genetic variation in the beta 3-adrenergic receptor and an increased capacity to gain weight in patients with morbid obesity. N Engl J Med 333(6):352–354
Honda K, Yamaguchi O, Nomiya M et al (2014) Association between polymorphism of Beta3-adrenoceptor gene and overactive bladder. Neurourol Urodyn 4:400–402
Ferreira CE, Fonseca AM, Silva ID et al (2011) The relationship between the Trp 64 Arg polymorphism of the beta 3-adrenoceptor gene and idiopathic overactive bladder. Am J Obstet Gynecol 205:82.e10–14
Ridley AJ, Hall A (1992) Distinct patterns of actin organization regulated by the small GTP-binding proteins Rac and Rho. Cold Spring Harb Symp Quant Biol 57:661–671
Loirand G, Guerin P, Pacaud P (2006) Rho kinases in cardiovascular physiology and pathophysiology. Circ Res 98(3):322–334
Somlyo AP, Somlyo AV (2000) Signal transduction by G-proteins, rho-kinase and protein phosphatase to smooth muscle and non-muscle myosin II. J Physiol 522(Pt 2):177–185
Amano M, Ito M, Kimura K et al (1996) Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase). J Biol Chem 271(34):20246–20249
Yoo S-Y, Kim J, Cheong S et al (2012) Rho-associated kinase 2 polymorphism in patients with vasospastic angina. Korean Circ J 42(6):406–413
Fukuhara S, Chikumi H, Gutkind JS (2001) RGS-containing RhoGEFs: the missing link between transforming G proteins and Rho? Oncogene 20(13):1661–1668
Bourne HR, Sanders DA, McCormick F (1990) The GTPase superfamily: a conserved switch for diverse cell functions. Nature 348(6297):125–132
Matsushita T, Ashikawa K, Yonemoto K et al (2010) Functional SNP of ARHGEF10 confers risk of atherothrombotic stroke. Hum Mol Genet 19(6):1137–1146
Zee RYL, Wang QM, Chasman DI et al (2014) Gene variations of ROCKs and risk of ischaemic stroke: the Women’s Genome Health Study. Clin Sci (Lond) 126(12):829–835
Hartmann KE, McPheeters ML, Biller DH et al (2009) Treatment of overactive bladder in women. Evid Rep Technol Assess (Full Rep) 187(1–120):v
Chancellor M, Boone T (2012) Anticholinergics for overactive bladder therapy: central nervous system effects. CNS Neurosci Ther 2:167–174
Tarcan T, Mangır N, Ozgur O et al (2012) OAB-V8 Asırı aktif mesane sorgulama formu validasyon calısmasi. Uroloji Bulteni 21:113–116
Kimura K, Sasaki N, Asano A et al (2000) Mutated human b3-adrenergic receptor (Trp64Arg) lowers the response to b3-adrenergic agonisnts in transfected 3T3-L1 preadipocytes. Horn Metab Res 32(3):91–96
Pietri-Rouxel F, St John Manning B, Gros J et al (1997) The biochemical effect of the naturally occurring Trp64 Arg mutation on human beta3-adrenoceptor activity. Eur J Biochem 247(3):1174–1179
Teitsma C, Rosette J, Michel M (2013) Are polymorphisms of the B3-adrenoceptor gene associated with an altered bladder function? Neurourol Urodyn 32:276–280
Qu HC, Zhang W, Liu YL et al (2015) Association between polymorphism of β3-adrenoceptor gene and overactive bladder: a meta-analysis. Genet Mol Res 14(1):2495–2501
Gurocak S, Konac E, Ure I et al (2015) The ımpact of gene polymorphisms on the success of anticholinergic treatment in children with overactive bladder. Dis Mark 2015:732686. https://doi.org/10.1155/2015/732686
Meekins AR, Murphy SK, Grenier C et al (2019) Role of β-3 adrenergic receptor polymorphism in overactive bladder. Neurourol Urodyn 38(5):1261–1265
Kandabashi T, Shimokawa H, Miyata K et al (2000) Inhibition of myosin phosphatase by upregulated rho-kinase plays a key role for coronary artery spasm in a porcine model with interleukin-1beta. Circulation 101(11):1319–1323
Mukai Y, Shimokawa H, Matoba T et al (2001) Involvement of Rho-kinase in hypertensive vascular disease: a novel therapeutic target in hypertension. FASEB J 6:1062–1064
Uehata M, Ishizaki T, Satoh H et al (1997) Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature 389(6654):990–994
Pandey P, Mohammad G, Singh Y, Pasha MA (2016) Polymorphisms and haplotype of ROCK2 associate with high altitude essential hypertension in native high altitude Ladakhi Indian population: a preliminary study. Clin Exp Hypertens 38(2):238–244
Liu L, Cao Y, Cui G et al (2013) Association analysis of polymorphisms in ROCK2 with cardiovascular disease in a chinese population. PLoS ONE ONE 8(1):e53905
Acknowledgements
This study was supported by the Pamukkale University Scientific Research Projects Department.
Funding
This study was funded by Pamukkale University Scientific Research Projects Department (Grant number 2017TIPF008).
Author information
Authors and Affiliations
Contributions
EF project development, data collection and management, data analysis, manuscript writing. ZA project development, data collection and management, manuscript editing. ŞA project development, data analysis. KK data collection. HA data analysis. HA project development, data management, data analysis, manuscript editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee (Pamukkale University Non-Interventional Clinical Research Ethics Committee; Reference number: 04; Date: 20.03.2017) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was taken from all human participants included in this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fırat, E., Aybek, Z., Akgün, Ş. et al. Relation of ADRB3, GEF, ROCK2 gene polymorphisms to clinical findings in overactive bladder. World J Urol 38, 2571–2575 (2020). https://doi.org/10.1007/s00345-019-03046-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-019-03046-5