Abstract
Purpose
This study aims to determine whether intra-network and inter-network brain connectivities are altered using an independent component analysis (ICA).
Methods
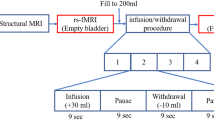
Resting-state functional MRI (rs-fMRI) data were acquired from 26 patients with OAB and 28 healthy controls (HC). Eleven resting-state networks (RSNs) were identified via ICA. General linear model (GLM) was used to compare intra-network FC and inter-network FC of RSNs between the two groups. Pearson correlation analyses were performed to investigate the relationship between the identified RSNs and clinical variables.
Results
Compared with HC, the OAB group showed abnormal FC within the sensorimotor-related network (SMN), the dorsal attention network (DAN), the dorsal visual network (dVN), and the left frontoparietal network (LFPN). With respect to inter-network interactions, decreased FC was detected between the SMN and the anterior default mode network (aDMN).
Conclusion
This study demonstrated that abnormal FC between RSNs may reflect the altered resting state of the brain–bladder network. The findings of this study provide complementary evidence that can help further understand the neural substrates of the overactive bladder.


Similar content being viewed by others
References
Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U, Van Kerrebroeck P, Victor A, Wein A (2003) The standardisation of terminology in lower urinary tract function: report from the standardisation sub-committee of the International Continence Society. Urology 61(1):37–49
Coyne KS, Sexton CC, Vats V, Thompson C, Kopp ZS, Milsom I (2011) National community prevalence of overactive bladder in the United States stratified by sex and age. Urology 77(5):1081–1087. https://doi.org/10.1016/j.urology.2010.08.039
Milsom I, Abrams P, Cardozo L, Roberts RG, Thuroff J, Wein AJ (2001) How widespread are the symptoms of an overactive bladder and how are they managed? A population-based prevalence study. BJU Int 87(9):760–766
Abrams P, Andersson KE, Birder L, Brubaker L, Cardozo L, Chapple C, Cottenden A, Davila W, de Ridder D, Dmochowski R, Drake M, Dubeau C, Fry C, Hanno P, Smith JH, Herschorn S, Hosker G, Kelleher C, Koelbl H, Khoury S, Madoff R, Milsom I, Moore K, Newman D, Nitti V, Norton C, Nygaard I, Payne C, Smith A, Staskin D, Tekgul S, Thuroff J, Tubaro A, Vodusek D, Wein A, Wyndaele JJ (2010) Fourth International Consultation on Incontinence Recommendations of the International Scientific Committee: evaluation and treatment of urinary incontinence, pelvic organ prolapse, and fecal incontinence. Neurourol Urodyn 29(1):213–240. https://doi.org/10.1002/nau.20870
Griffiths D, Derbyshire S, Stenger A, Resnick N (2005) Brain control of normal and overactive bladder. J Urol 174(5):1862–1867. https://doi.org/10.1097/01.ju.0000177450.34451.97
Griffiths D, Tadic SD (2008) Bladder control, urgency, and urge incontinence: evidence from functional brain imaging. Neurourol Urodyn 27(6):466–474. https://doi.org/10.1002/nau.20549
Griffiths DJ (2011) Use of functional imaging to monitor central control of voiding in humans. Handb Exp Pharmacol 202:81–97. https://doi.org/10.1007/978-3-642-16499-6_5
Du Y, Fan Y (2013) Group information guided ICA for fMRI data analysis. NeuroImage 69:157–197. https://doi.org/10.1016/j.neuroimage.2012.11.008
Beckmann CF, DeLuca M, Devlin JT, Smith SM (2005) Investigations into resting-state connectivity using independent component analysis. Philos Trans R Soc Lond B Biol Sci 360(1457):1001–1013. https://doi.org/10.1098/rstb.2005.1634
Gad PN, Roy RR, Zhong H, Gerasimenko YP, Taccola G, Edgerton VR (2016) Neuromodulation of the neural circuits controlling the lower urinary tract. Exp Neurol 285:182–189
Rickenbacher E, Baez MA, Hale L, Leiser SC, Zderic SA, Valentino RJ (2008) Impact of overactive bladder on the brain: central sequelae of a visceral pathology. Proc Natl Acad Sci USA 105(30):10589–10594. https://doi.org/10.1073/pnas.0800969105
Nour S, Svarer C, Kristensen JK, Paulson OB, Law I (2000) Cerebral activation during micturition in normal men. Brain 123(Pt 4):781–789
Spreng RN, Stevens WD, Chamberlain JP, Gilmore AW, Schacter DL (2010) Default network activity, coupled with the frontoparietal control network, supports goal-directed cognition. NeuroImage 53(1):303–317. https://doi.org/10.1016/j.neuroimage.2010.06.016
Spreng RN, DuPre E, Selarka D, Garcia J, Gojkovic S, Mildner J, Luh WM (2014) Goal-congruent default network activity facilitates cognitive control. J Neurosci 34(42):14108–14114. https://doi.org/10.1523/jneurosci.2815-14.2014
Xu X, Yuan H, Lei X (2016) Activation and connectivity within the default mode network contribute independently to future-oriented thought. Sci Rep 6:21001. https://doi.org/10.1038/srep21001
Blok BF (2002) Central pathways controlling micturition and urinary continence. Urology 59(5 Suppl 1):13–17
Griffiths D, Tadic SD, Schaefer W, Resnick NM (2007) Cerebral control of the bladder in normal and urge-incontinent women. NeuroImage 37:1–7
Tadic SD, Griffiths D, Schaefer W, Resnick NM (2008) Abnormal connections in the supraspinal bladder control network in women with urge urinary incontinence. NeuroImage 39:1647–1653
Dhond RP, Yeh C, Park K, Kettner N, Napadow V (2008) Acupuncture modulates resting state connectivity in default and sensorimotor brain networks. Pain 136(3):407–418. https://doi.org/10.1016/j.pain.2008.01.011
Power Jonathan D, Cohen Alexander L, Nelson Steven M, Wig Gagan S, Barnes Kelly A, Church Jessica A, Vogel Alecia C, Laumann Timothy O, Miezin Fran M, Schlaggar Bradley L, Petersen Steven E (2011) Functional network organization of the human brain. Neuron 72(4):665–678. https://doi.org/10.1016/j.neuron.2011.09.006
Duncan J (2010) The multiple-demand (MD) system of the primate brain: mental programs for intelligent behaviour. Trends Cogn Sci 14(4):172–179. https://doi.org/10.1016/j.tics.2010.01.004
Corbetta M, Shulman GL (2002) Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci 3(3):201
Dosenbach NUF, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach RAT, Fox MD, Snyder AZ, Vincent JL, Raichle ME, Schlaggar BL, Petersen SE (2007) Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci 104(26):11073–11078. https://doi.org/10.1073/pnas.0704320104
Migliaccio R, Gallea C, Kas A, Perlbarg V, Samri D, Trotta L, Michon A, Lacomblez L, Dubois B, Lehericy S, Bartolomeo P (2016) Functional connectivity of ventral and dorsal visual streams in posterior cortical atrophy. J Alzheimer’s Dis 51(4):1119–1130. https://doi.org/10.3233/jad-150934
Goodale MA, Milner AD (1992) Separate visual pathways for perception and action. Trends Neurosci 15(1):20–25
Tadic SD, Holstege G, Griffiths DJ (2012) The CNS and bladder dysfunction. F1000 Med Reps 4:20. https://doi.org/10.3410/m4-20
Funding
This study was supported by grants from the National Nature Science Foundation of China (No. Grant nos. 81541129, 81301016, KZ73100001) and the Beihang University Research Fund Program under Project ZG216S1871.
Author information
Authors and Affiliations
Contributions
LZ: data collection or management, data analysis, manuscript writing and editing. JC: data analysis, manuscript writing and editing. SW: protocol/project development. YZ: data collection or management. BW: data collection or management. HG: protocol/project development.
Corresponding author
Ethics declarations
Conflict of interest
All the authors declare that they have no conflict of interest to declare.
Research involving human participants and/or animals
Human participants: yes; animals: no. This study was approved by the Committee for Human Research in Beijing Chaoyang Hospital and followed by all regulations (Grant nos. 2015-ke-21).
Informed consent
All the patients have provided informed consent.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zuo, L., Chen, J., Wang, S. et al. Intra- and inter-resting-state networks abnormalities in overactive bladder syndrome patients: an independent component analysis of resting-state fMRI. World J Urol 38, 1027–1034 (2020). https://doi.org/10.1007/s00345-019-02838-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-019-02838-z