Abstract
Peyronie’s disease (PD) is a superficial fibrosing disorder that causes penile deformity and can interfere with sexual intercourse and reproduction, as well as diminish quality of life. While the exact mechanism of PD is still being investigated, there is likely a genetic component to the predisposition to penile plaque formation. Ultimately, however, perturbations in normal wound healing and aberrant deposition of extracellular matrix components lead to fibrotic tissue deposition. Fibrosis in PD is regulated by a complex pathway of inflammatory and fibrotic mediators. Currently there are no treatments for PD that address an underlying cause or disease progression. In this review, we provide an overview of the known inflammatory and fibrotic mediators of PD and explore the pathophysiology of other human superficial fibrosing disorders to develop further insights into PD.


Similar content being viewed by others
References
Dibenedetti DB, Nguyen D, Zografos L, Ziemiecki R, Zhou X (2011) A population-based study of Peyronie’s disease: prevalence and treatment patterns in the United States. Adv Urol 2011:282503. https://doi.org/10.1155/2011/282503
Stuntz M, Perlaky A, Des Vignes F, Kyriakides T, Glass D (2016) The prevalence of Peyronie’s disease in the United States: a population-based study. PLoS One 11(2):e0150157. https://doi.org/10.1371/journal.pone.0150157
Schwarzer U, Sommer F, Klotz T, Braun M, Reifenrath B, Engelmann U (2001) The prevalence of Peyronie’s disease: results of a large survey. BJU Int 88(7):727–730
Lindsay MB, Schain DM, Grambsch P, Benson RC, Beard CM, Kurland LT (1991) The incidence of Peyronie’s disease in Rochester, Minnesota, 1950 through 1984. J Urol 146(4):1007–1009
Gonzalez-Cadavid NF, Rajfer J (2005) Mechanisms of disease: new insights into the cellular and molecular pathology of Peyronie’s disease. Nat Clin Pract Urol 2(6):291–297. https://doi.org/10.1038/ncpuro0201
Nyberg LM Jr, Bias WB, Hochberg MC, Walsh PC (1982) Identification of an inherited form of Peyronie’s disease with autosomal dominant inheritance and association with Dupuytren’s contracture and histocompatibility B7 cross-reacting antigens. J Urol 128(1):48–51
Willscher MK, Cwazka WF, Novicki DE (1979) The association of histocompatibility antigens of the B7 cross-reacting group with Peyronie’s disease. J Urol 122(1):34–35
Rockey DC, Bell PD, Hill JA (2015) Fibrosis—a common pathway to organ injury and failure. N Engl J Med 373(1):96. https://doi.org/10.1056/NEJMc1504848
Rosenbloom J, Castro SV, Jimenez SA (2010) Narrative review: fibrotic diseases: cellular and molecular mechanisms and novel therapies. Ann Intern Med 152(3):159–166. https://doi.org/10.7326/0003-4819-152-3-201002020-00007
Dibenedetti DB, Nguyen D, Zografos L, Ziemiecki R, Zhou X (2011) Prevalence, incidence, and treatments of Dupuytren’s disease in the United States: results from a population-based study. Hand (New York, NY) 6(2):149–158. https://doi.org/10.1007/s11552-010-9306-4
Nugteren HM, Nijman JM, de Jong IJ, van Driel MF (2011) The association between Peyronie’s and Dupuytren’s disease. Int J Impot Res 23(4):142–145. https://doi.org/10.1038/ijir.2011.18
Shindel AW, Sweet G, Thieu W, Durbin-Johnson B, Rothschild J, Szabo R (2017) Prevalence of Peyronie’s disease-like symptoms in men presenting with dupuytren contractures. Sex Med 5(3):e135–e141. https://doi.org/10.1016/j.esxm.2017.06.001
Alioto RJ, Rosier RN, Burton RI, Puzas JE (1994) Comparative effects of growth factors on fibroblasts of Dupuytren’s tissue and normal palmar fascia. J Hand Surg 19(3):442–452
Badalamente MA, Hurst LC, Grandia SK, Sampson SP (1992) Platelet-derived growth factor in Dupuytren’s disease. J Hand Surg 17(2):317–323
Badalamente MA, Sampson SP, Hurst LC, Dowd A, Miyasaka K (1996) The role of transforming growth factor beta in Dupuytren’s disease. J Hand Surg 21(2):210–215. https://doi.org/10.1016/s0363-5023(96)80102-x
Bayat A, Alansar A, Hajeer AH, Shah M, Watson JS, Stanley JK, Ferguson MW, Ollier WE (2002) Genetic susceptibility in Dupuytren’s disease: lack of association of a novel transforming growth factor beta(2) polymorphism in Dupuytren’s disease. J Hand Surg (Edinburgh, Scotland) 27(1):47–49. https://doi.org/10.1054/jhsb.2001.0689
Berndt A, Kosmehl H, Mandel U, Gabler U, Luo X, Celeda D, Zardi L, Katenkamp D (1995) TGF beta and bFGF synthesis and localization in Dupuytren’s disease (nodular palmar fibromatosis) relative to cellular activity, myofibroblast phenotype and oncofetal variants of fibronectin. Histochem J 27(12):1014–1020
Gonzalez AM, Buscaglia M, Fox R, Isacchi A, Sarmientos P, Farris J, Ong M, Martineau D, Lappi DA, Baird A (1992) Basic fibroblast growth factor in Dupuytren’s contracture. Am J Pathol 141(3):661–671
Qian A, Meals RA, Rajfer J, Gonzalez-Cadavid NF (2004) Comparison of gene expression profiles between Peyronie’s disease and Dupuytren’s contracture. Urology 64(2):399–404. https://doi.org/10.1016/j.urology.2004.04.006
Bilgutay AN, Pastuszak AW (2015) Peyronie’s disease: a review of etiology, diagnosis, and management. Curr Sex Health Rep 7(2):117–131. https://doi.org/10.1007/s11930-015-0045-y
Kim SK, Ioannidis JPA, Ahmed MA, Avins AL, Kleimeyer JP, Fredericson M, Dragoo JL (2018) Two genetic variants associated with plantar fascial disorders. Int J Sports Med 39(4):314–321. https://doi.org/10.1055/s-0044-100280
Dolmans GH, de Bock GH, Werker PM (2012) Dupuytren diathesis and genetic risk. J Hand Surg 37(10):2106–2111. https://doi.org/10.1016/j.jhsa.2012.07.017
Martinez MA, Ferrando D, Cordero PJ (1997) Idiopathic pulmonary fibrosis and Peyronie’s disease. Arch Bronconeumol 33(10):549–550
Lyles KW, Gold DT, Newton RA, Parekh S, Shipp KM, Pieper CF, Krishan R, Carson CC 3rd (1997) Peyronie’s disease is associated with Paget’s disease of bone. J Bone Miner Res 12(6):929–934. https://doi.org/10.1359/jbmr.1997.12.6.929
Akbal C, Tanidir Y, Ozgen MB, Simsek F (2008) Erectile dysfunction and Peyronie’s disease in patient with retroperitoneal fibrosis. Int Urol Nephrol 40(4):971–975. https://doi.org/10.1007/s11255-008-9381-4
Chen TY, Zahran AR, Carrier S (2001) Penile curvature associated with scleroderma. Urology 58(2):282
Chen DL, Chong AH, Green J, Orchard D, Williams R, Clemens L (2006) A novel case of polyfibromatosis and interstitial granulomatous dermatitis with arthritis. J Am Acad Dermatol 55(2 Suppl):S32–S37. https://doi.org/10.1016/j.jaad.2006.02.038
Simeon CP, Fonollosa V, Vilardell M, Ordi J, Solans R, Lima J (1994) Impotence and Peyronie’s disease in systemic sclerosis. Clin Exp Rheumatol 12(4):464
Ordi J, Selva A, Fonollosa V, Vilardell M, Jordana R, Tolosa C (1990) Peyronie’s disease in systemic sclerosis. Ann Rheum Dis 49(2):134–135
Ventimiglia E, Capogrosso P, Colicchia M, Boeri L, Serino A, La Croce G, Russo A, Capitanio U, Briganti A, Cantiello F, Mirone V, Damiano R, Montorsi F, Salonia A (2015) Peyronie’s disease and autoimmunity-a real-life clinical study and comprehensive review. J Sex Med 12(4):1062–1069. https://doi.org/10.1111/jsm.12825
Pastuszak AW, Rodriguez KM, Solomon ZJ, Kohn TP, Lipshultz LI, Eisenberg ML (2018) Increased risk of incident disease in men with Peyronie’s disease: analysis of U.S. claims data. J Sex Med 15(6):894–901. https://doi.org/10.1016/j.jsxm.2018.04.640
Lue TF (2002) Peyronie’s disease: an anatomically-based hypothesis and beyond. Int J Impot Res 14(5):411–413. https://doi.org/10.1038/sj.ijir.3900876
Meng XM, Nikolic-Paterson DJ, Lan HY (2016) TGF-beta: the master regulator of fibrosis. Nat Rev Nephrol 12(6):325–338. https://doi.org/10.1038/nrneph.2016.48
Wynn TA (2008) Cellular and molecular mechanisms of fibrosis. J Pathol 214(2):199–210. https://doi.org/10.1002/path.2277
Border WA, Noble NA (1994) Transforming growth factor beta in tissue fibrosis. N Engl J Med 331(19):1286–1292. https://doi.org/10.1056/nejm199411103311907
Border WA, Ruoslahti E (1992) Transforming growth factor-beta in disease: the dark side of tissue repair. J Clin Investig 90(1):1–7. https://doi.org/10.1172/jci115821
El-Sakka AI, Hassoba HM, Chui RM, Bhatnagar RS, Dahiya R, Lue TF (1997) An animal model of Peyronie’s-like condition associated with an increase of transforming growth factor beta mRNA and protein expression. J Urol 158(6):2284–2290
El-Sakka AI, Hassoba HM, Pillarisetty RJ, Dahiya R, Lue TF (1997) Peyronie’s disease is associated with an increase in transforming growth factor-beta protein expression. J Urol 158(4):1391–1394
Ryu JK, Kim WJ, Choi MJ, Park JM, Song KM, Kwon MH, Das ND, Kwon KD, Batbold D, Yin GN, Suh JK (2013) Inhibition of histone deacetylase 2 mitigates profibrotic TGF-beta1 responses in fibroblasts derived from Peyronie’s plaque. Asian J Androl 15(5):640–645. https://doi.org/10.1038/aja.2013.61
Gonzalez-Cadavid NF, Magee TR, Ferrini M, Qian A, Vernet D, Rajfer J (2002) Gene expression in Peyronie’s disease. Int J Impot Res 14(5):361–374. https://doi.org/10.1038/sj.ijir.3900873
Giannandrea M, Parks WC (2014) Diverse functions of matrix metalloproteinases during fibrosis. Dis Models Mech 7(2):193–203. https://doi.org/10.1242/dmm.012062
Pardo A, Selman M (2006) Matrix metalloproteases in aberrant fibrotic tissue remodeling. Proc Am Thorac Soc 3(4):383–388. https://doi.org/10.1513/pats.200601-012TK
Van de Water L (1997) Mechanisms by which fibrin and fibronectin appear in healing wounds: implications for Peyronie’s disease. J Urol 157(1):306–310
El-Sakka AI, Salabas E, Dincer M, Kadioglu A (2013) The pathophysiology of Peyronie’s disease. Arab J Urol 11(3):272–277. https://doi.org/10.1016/j.aju.2013.06.006
Moreland RB, Nehra A (2002) Pathophysiology of Peyronie’s disease. Int J Impot Res 14(5):406–410. https://doi.org/10.1038/sj.ijir.3900875
Liu RM, Desai LP (2015) Reciprocal regulation of TGF-beta and reactive oxygen species: a perverse cycle for fibrosis. Redox Biol 6:565–577. https://doi.org/10.1016/j.redox.2015.09.009
Vernet D, Nolazco G, Cantini L, Magee TR, Qian A, Rajfer J, Gonzalez-Cadavid NF (2005) Evidence that osteogenic progenitor cells in the human tunica albuginea may originate from stem cells: implications for peyronie disease. Biol Reprod 73(6):1199–1210. https://doi.org/10.1095/biolreprod.105.041038
Huleihel M, Douvdevani A, Segal S, Apte RN (1990) Regulation of interleukin 1 generation in immune-activated fibroblasts. Eur J Immunol 20(4):731–738. https://doi.org/10.1002/eji.1830200404
Pryor JP, Ralph DJ (2002) Clinical presentations of Peyronie’s disease. Int J Impot Res 14(5):414–417. https://doi.org/10.1038/sj.ijir.3900877
Sasaki K, Hattori T, Fujisawa T, Takahashi K, Inoue H, Takigawa M (1998) Nitric oxide mediates interleukin-1-induced gene expression of matrix metalloproteinases and basic fibroblast growth factor in cultured rabbit articular chondrocytes. J Biochem 123(3):431–439
Bivalacqua TJ, Champion HC, Hellstrom WJ (2002) Implications of nitric oxide synthase isoforms in the pathophysiology of Peyronie’s disease. Int J Impot Res 14(5):345–352. https://doi.org/10.1038/sj.ijir.3900872
Mulhall JP, Thom J, Lubrano T, Shankey TV (2001) Basic fibroblast growth factor expression in Peyronie’s disease. J Urol 165(2):419–423. https://doi.org/10.1097/00005392-200102000-00016
Lambert E, Dasse E, Haye B, Petitfrere E (2004) TIMPs as multifacial proteins. Crit Rev Oncol/Hematol 49(3):187–198. https://doi.org/10.1016/j.critrevonc.2003.09.008
Davila HH, Magee TR, Zuniga FI, Rajfer J, Gonzalez-Cadavid NF (2005) Peyronie’s disease associated with increase in plasminogen activator inhibitor in fibrotic plaque. Urology 65(4):645–648. https://doi.org/10.1016/j.urology.2005.01.010
Higgins DF, Kimura K, Bernhardt WM, Shrimanker N, Akai Y, Hohenstein B, Saito Y, Johnson RS, Kretzler M, Cohen CD, Eckardt KU, Iwano M, Haase VH (2007) Hypoxia promotes fibrogenesis in vivo via HIF-1 stimulation of epithelial-to-mesenchymal transition. J Clin Investig 117(12):3810–3820. https://doi.org/10.1172/jci30487
Sanchez-Elsner T, Botella LM, Velasco B, Corbi A, Attisano L, Bernabeu C (2001) Synergistic cooperation between hypoxia and transforming growth factor-beta pathways on human vascular endothelial growth factor gene expression. J Biol Chem 276(42):38527–38535. https://doi.org/10.1074/jbc.M104536200
Amara N, Goven D, Prost F, Muloway R, Crestani B, Boczkowski J (2010) NOX4/NADPH oxidase expression is increased in pulmonary fibroblasts from patients with idiopathic pulmonary fibrosis and mediates TGFbeta1-induced fibroblast differentiation into myofibroblasts. Thorax 65(8):733–738. https://doi.org/10.1136/thx.2009.113456
Sancho P, Mainez J, Crosas-Molist E, Roncero C, Fernandez-Rodriguez CM, Pinedo F, Huber H, Eferl R, Mikulits W, Fabregat I (2012) NADPH oxidase NOX4 mediates stellate cell activation and hepatocyte cell death during liver fibrosis development. PLoS One 7(9):e45285. https://doi.org/10.1371/journal.pone.0045285
Poli G, Parola M (1997) Oxidative damage and fibrogenesis. Free Radic Biol Med 22(1–2):287–305
Paulis G, Romano G, Paulis L, Barletta D (2017) Recent pathophysiological aspects of Peyronie’s disease: role of free radicals, rationale, and therapeutic implications for antioxidant treatment-literature review. Adv Urol 2017:4653512. https://doi.org/10.1155/2017/4653512
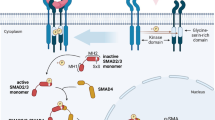
Derynck R, Zhang YE (2003) Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature 425(6958):577–584. https://doi.org/10.1038/nature02006
Miyazono K, Kusanagi K, Inoue H (2001) Divergence and convergence of TGF-beta/BMP signaling. J Cell Physiol 187(3):265–276. https://doi.org/10.1002/jcp.1080
Zeisberg M, Hanai J, Sugimoto H, Mammoto T, Charytan D, Strutz F, Kalluri R (2003) BMP-7 counteracts TGF-beta1-induced epithelial-to-mesenchymal transition and reverses chronic renal injury. Nat Med 9(7):964–968. https://doi.org/10.1038/nm888
Chen G, Deng C, Li YP (2012) TGF-beta and BMP signaling in osteoblast differentiation and bone formation. Int J Biol Sci 8(2):272–288. https://doi.org/10.7150/ijbs.2929
Nohe A, Keating E, Knaus P, Petersen NO (2004) Signal transduction of bone morphogenetic protein receptors. Cell Signal 16(3):291–299
Jinnin M (2010) Mechanisms of skin fibrosis in systemic sclerosis. J Dermatol 37(1):11–25. https://doi.org/10.1111/j.1346-8138.2009.00738.x
Dolmans GH, Werker PM, de Jong IJ, Nijman RJ, Wijmenga C, Ophoff RA (2012) WNT2 locus is involved in genetic susceptibility of Peyronie’s disease. J Sex Med 9(5):1430–1434. https://doi.org/10.1111/j.1743-6109.2012.02704.x
Kavian N, Servettaz A, Weill B, Batteux F (2012) New insights into the mechanism of notch signalling in fibrosis. Open Rheumatol J 6:96–102. https://doi.org/10.2174/1874312901206010096
Dees C, Tomcik M, Zerr P, Akhmetshina A, Horn A, Palumbo K, Beyer C, Zwerina J, Distler O, Schett G, Distler JH (2011) Notch signalling regulates fibroblast activation and collagen release in systemic sclerosis. Ann Rheum Dis 70(7):1304–1310. https://doi.org/10.1136/ard.2010.134742
Rohatgi R, Milenkovic L, Corcoran RB, Scott MP (2009) Hedgehog signal transduction by Smoothened: pharmacologic evidence for a 2-step activation process. Proc Natl Acad Sci USA 106(9):3196–3201. https://doi.org/10.1073/pnas.0813373106
Horn A, Palumbo K, Cordazzo C, Dees C, Akhmetshina A, Tomcik M, Zerr P, Avouac J, Gusinde J, Zwerina J, Roudaut H, Traiffort E, Ruat M, Distler O, Schett G, Distler JH (2012) Hedgehog signaling controls fibroblast activation and tissue fibrosis in systemic sclerosis. Arthritis Rheum 64(8):2724–2733. https://doi.org/10.1002/art.34444
Clevers H, Nusse R (2012) Wnt/beta-catenin signaling and disease. Cell 149(6):1192–1205. https://doi.org/10.1016/j.cell.2012.05.012
Beyer C, Schramm A, Akhmetshina A, Dees C, Kireva T, Gelse K, Sonnylal S, de Crombrugghe B, Taketo MM, Distler O, Schett G, Distler JH (2012) beta-catenin is a central mediator of pro-fibrotic Wnt signaling in systemic sclerosis. Ann Rheum Dis 71(5):761–767. https://doi.org/10.1136/annrheumdis-2011-200568
Acknowledgements
A.W.P. is a National Institutes of Health K08 Scholar supported by a Mentored Career Development Award (K08DK115835-01) from the National Institute of Diabetes and Digestive and Kidney Diseases. This work is also supported in part through a Urology Care Foundation Rising Stars in Urology Award (to A.W.P.).
Author information
Authors and Affiliations
Contributions
DPP: literature review, manuscript writing/editing, critical review, approval of final manuscript. MBC: literature review, manuscript writing/editing, critical review, approval of final manuscript. JMH: manuscript writing/editing, critical review, approval of final manuscript. AWP: literature review, manuscript writing/editing, critical review, approval of final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
DP Patel: None. MB Christensen: None. JM Hotaling: Dr. Hotaling receives research and fellowship support from Boston Scientific and Endo Pharmaceuticals. He holds leadership roles/equity interest in Nanonc, StreamDx, and Andro360 which are all early stage startup companies. AW Pastuszak: Dr. Pastuszak is an advisor to Endo Pharmaceuticals, Boston Scientific, and Antares Pharmaceuticals, and a speaker for Endo Pharmaceuticals and Bayer AG. He is a consultant and receives research and fellowship support from Endo Pharmaceuticals. He holds a leadership position with Woven Health, of which he is also a founder. As this is a review article, no research involving human participants and/or animals was carried out and no informed consent was needed.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Patel, D.P., Christensen, M.B., Hotaling, J.M. et al. A review of inflammation and fibrosis: implications for the pathogenesis of Peyronie’s disease. World J Urol 38, 253–261 (2020). https://doi.org/10.1007/s00345-019-02815-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-019-02815-6