Abstract
Purpose
Early tumor shrinkage (eTS) has prognostic value in metastatic renal cell carcinoma (mRCC). We aimed to validate the role of eTS in first line treatment from the COMPARZ study (NCT00720941).
Methods
1100 patients treated with sunitinib or pazopanib were analyzed for tumor response according to RECIST 1.0. eTS was defined as tumor shrinkage by ≥ 10%. A landmark analysis was performed on day (d) 42 and 90 and Cox proportional hazards regression was computed for the prognostic effect of eTS.
Results
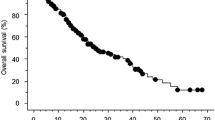
In patients with eTS median OS was 34.1 [CI 95% 28.4; not reached (NR)] and 33.6 (CI 95% 30.1; NR) months (mo) at d 42 and 90, respectively, compared to 19.6 (CI 95% 14.0; 28.9) and 15.1 (CI 95% 12.4; 18.7) mo for patients without eTS. There was no interaction between type of treatment and eTS (d 42 p = 0.79; d 90 p = 0.37). eTS ≥ 10% remained an independent prognostic marker in multivariable analyses at both d 42 and 90.
Conclusions
Similar results were found for eTS at the 42 and 90 days landmarks. eTS ≥ 10% has prognostic relevance in mRCC and reflects a putative tool to guide future clinical treatment.

Similar content being viewed by others
References
Motzer RJ, Hutson TE, Cella D, Reeves J, Hawkins R, Guo J et al (2013) Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N Engl J Med 369:722–731. https://doi.org/10.1056/NEJMoa1303989
Grünwald V, McKay RR, Krajewski KM, Kalanovic D, Lin X, Perkins JJ et al (2015) Depth of remission is a prognostic factor for survival in patients with metastatic renal cell carcinoma. Eur Urol 67:952–958. https://doi.org/10.1016/j.eururo.2014.12.036
Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S et al (2015) Nivolumab versus everolimus in advanced renal-cell carcinoma 373:1803–1813. https://doi.org/10.1056/NEJMoa1510665
Motzer RJ, Hutson TE, Glen H, Michaelson MD, Molina A, Eisen T et al (2015) Lenvatinib, everolimus, and the combination in patients with metastatic renal cell carcinoma: a randomised, phase 2, open-label, multicentre trial. Lancet Oncol 16:1473–1482. https://doi.org/10.1016/S1470-2045(15)00290-9
Choueiri TK, Escudier B, Powles T, Mainwaring PN, Rini BI, Donskov F et al (2015) Cabozantinib versus everolimus in advanced renal-cell carcinoma. N Engl J Med 373:1814–1823. https://doi.org/10.1056/NEJMoa1510016
Grünwald V, Lin X, Kalanovic D, Simantov R (2016) Early tumour shrinkage: a tool for the detection of early clinical activity in metastatic renal cell carcinoma. Eur Urol 70:1006–1015. https://doi.org/10.1016/j.eururo.2016.05.010
Ko JJ, Choueiri TK, Rini BI, Lee JL, Kroeger N, Srinivas S et al (2014) First-, second-, third-line therapy for mRCC: benchmarks for trial design from the IMDC. Br J Cancer 110:1917–1922. https://doi.org/10.1038/bjc.2014.25
Escudier B, Porta C, Schmidinger M, Rioux-Leclercq N, Bex A, Khoo V et al (2016) Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 27:v58–v68. https://doi.org/10.1093/annonc/mdw328
Powles T, Staehler M, Ljungberg B, Bensalah K, Canfield SE, Dabestani S et al (2016) European association of urology guidelines for clear cell renal cancers that are resistant to vascular endothelial growth factor receptor-targeted therapy. Eur Urol. https://doi.org/10.1016/j.eururo.2016.06.009
Doehn C, Grünwald V, Steiner T, Follmann M, Rexer H, Krege S (2016) The diagnosis, treatment, and follow-up of renal cell carcinoma. Dtsch Arztebl Int 113:590–596. https://doi.org/10.3238/arztebl.2016.0590
Calvo E, Schmidinger M, Heng DYC, Grünwald V, Escudier B (2016) Improvement in survival end points of patients with metastatic renal cell carcinoma through sequential targeted therapy. Cancer Treat Rev 50:109–117. https://doi.org/10.1016/j.ctrv.2016.09.002
Ornstein MC, Wood LS, Elson P, Allman KD, Beach J, Martin A et al (2017) A phase II study of intermittent sunitinib in previously untreated patients with metastatic renal cell carcinoma. J Clin Oncol 35:1764–1769. https://doi.org/10.1200/JCO.2016.71.1184
Collinson FJ, Gregory WM, McCabe C, Howard H, Lowe C, Potrata D et al (2012) The STAR trial protocol: a randomised multi-stage phase II/III study of sunitinib comparing temporary cessation with allowing continuation, at the time of maximal radiological response, in the first-line treatment of locally advanced/metastatic renal cancer. BMC Cancer 12:598. https://doi.org/10.1186/1471-2407-12-598
Abel EJ, Culp SH, Tannir NM, Tamboli P, Matin SF, Wood CG (2011) Early primary tumor size reduction is an independent predictor of improved overall survival in metastatic renal cell carcinoma patients treated with sunitinib. Eur Urol 60:1–7. https://doi.org/10.1016/j.eururo.2011.07.008
Krajewski KM, Guo M, van den Abbeele AD, Yap J, Ramaiya N, Jagannathan J et al (2011) Comparison of four early post therapy imaging changes (EPTIC; RECIST 1.0, tumor shrinkage, computed tomography tumor density, Choi criteria) in assessing outcome to vascular endothelial growth factor-targeted therapy in patients with advanced renal cell carcinoma. Eur Urol 59:856–862. https://doi.org/10.1016/j.eururo.2011.01.038
Busch J, Seidel C, Goranova I, Erber B, Peters R, Friedersdorff F et al (2014) Categories of response to first line vascular endothelial growth factor receptor targeted therapy and overall survival in patients with metastatic renal cell carcinoma. Eur J Cancer 50:563–569. https://doi.org/10.1016/j.ejca.2013.10.017
Motzer RJ, Sharma P, Escudier BJ, McDermott DF, George S, Srinivas S et al (2016) Correlation of response with overall survival (OS) for nivolumab vs everolimus in advanced renal cell carcinoma (aRCC): results from the phase III CheckMate 025 study. J Clin Oncol 34:4552. https://doi.org/10.1200/JCO.2016.34.15_suppl.4552
McDermott DF, Drake CG, Sznol M, Choueiri TK, Powderly JD, Smith DC et al (2015) Survival, durable response, and long-term safety in patients with previously treated advanced renal cell carcinoma receiving nivolumab. J Clin Oncol 33:2013–2020. https://doi.org/10.1200/JCO.2014.58.1041
Krajewski KM, Nishino M, Ramaiya NH, Choueiri TK (2015) RECIST 1.1 compared with RECIST 1.0 in patients with advanced renal cell carcinoma receiving vascular endothelial growth factor-targeted therapy. AJR Am J Roentgenol 204:W282–W288. https://doi.org/10.2214/AJR.14.13236
Acknowledgements
The authors thank the patients and investigators who participated in the COMPARZ trial used for this analysis.
Author information
Authors and Affiliations
Contributions
VG had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: VG, GP. Acquisition of data: VG, MD, GP. Analysis and interpretation of data: VG, MD, GP. Drafting of the manuscript: VG. Critical revision of the manuscript for important intellectual content: VG, MD, GP. Statistical analysis: GP. Administrative, technical, or material support: Not applicable. Supervision: VG.
Corresponding author
Ethics declarations
Research support
The study sponsor (Novartis) shared access to the primary data of the COMPARZ study but was not involved in concept, design, conduct, or analysis of the data. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interest
Viktor Grünwald has an advisory role at Pfizer, Novartis, Bristol Myer Squibb, Ipsen, Eisai, Roche and has received honoraria from Pfizer, Novartis, Bristol Myer Squibb, Ipsen, Eisai and Roche. Marion Dietrich and Gregory Pond do not have any conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
345_2018_2297_MOESM1_ESM.docx
Supplementary material 1 Figure A.1: Martingale Residual Plot for percentage Shrinkage as a prognostic factor for OS A) at day 42 and B) day 90, indicating 10% as a reasonable cutpoint for tumor shrinkage at both time points. (DOCX 128 kb)
345_2018_2297_MOESM2_ESM.pptx
Supplementary material 2 Figure A.2: Consort diagram of patients included into the trial. Patients not available for response assessment at day 42 and 90 are depicted. (PPTX 37 kb)
Rights and permissions
About this article
Cite this article
Grünwald, V., Dietrich, M. & Pond, G.R. Early tumor shrinkage is independently associated with improved overall survival among patients with metastatic renal cell carcinoma: a validation study using the COMPARZ cohort. World J Urol 36, 1423–1429 (2018). https://doi.org/10.1007/s00345-018-2297-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-018-2297-4