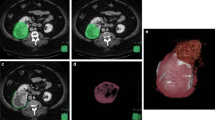
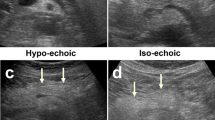
Abstract
Objective
To assess the performance of computed tomography (CT) texture analysis to predict the presence of adherent perinephric fat (APF).
Materials and methods
Seventy patients with small renal tumors treated with robot-assisted partial nephrectomy were included. Patients were divided into two groups according to the presence of APF. We extracted 15 image features from unenhanced CT and contrast-enhanced CT corresponding to first-order and second-order Haralick textural features. Predictors of APF were evaluated by univariable and multivariable analysis. Receiver operating characteristic (ROC) analysis was performed and the area under the ROC curve (AUC) to predict APF was calculated for the independent predictors.
Results
APF was observed in 26 patients (37%). We identified entropy (p = 0.01), sum entropy (p = 0.02) and difference entropy (p = 0.05) as significant independent predictors of APF. In the portal phase, we identified correlation (p = 0.03), inverse difference moment (p = 0.01), sum entropy (p = 0.02), entropy (p = 0.01), difference variance (p = 0.04) and difference entropy (p = 0.02) as significant independent predictors of APF. Combining these parameters yielded to an ROC-AUC of 0.82 (95% CI 0.65–0.86).
Conclusion
Results from this preliminary study suggest that CT texture analysis might be a promising quantitative imaging tool that helps urologist to identify APF.




Similar content being viewed by others
References
Patard J-J, Rodriguez A, Rioux-Leclercq N, Guillé F, Lobel B (2002) Prognostic significance of the mode of detection in renal tumours. BJU Int 90(4):358–363
Ljungberg B, Bensalah K, Canfield S, Dabestani S, Hofmann F, Hora M et al (2015) EAU guidelines on renal cell carcinoma: 2014 update. Eur Urol 67(5):913–924
Simhan J, Smaldone MC, Tsai KJ, Canter DJ, Li T, Kutikov A et al (2011) Objective measures of renal mass anatomic complexity predict rates of major complications following partial nephrectomy. Eur Urol 60(4):724–730
Mari A, Antonelli A, Bertolo R, Bianchi G, Borghesi M, Ficarra V et al (2017) Predictive factors of overall and major postoperative complications after partial nephrectomy: results from a multicenter prospective study (The RECORd 1 project). Eur J Surg Oncol EJSO 43(4):823–830
Kocher NJ, Kunchala S, Reynolds C, Lehman E, Nie S, Raman JD (2016) Adherent perinephric fat at minimally invasive partial nephrectomy is associated with adverse peri-operative outcomes and malignant renal histology. BJU Int 117(4):636–641
Khene Z-E, Peyronnet B, Mathieu R, Fardoun T, Verhoest G, Bensalah K (2015) Analysis of the impact of adherent perirenal fat on peri-operative outcomes of robotic partial nephrectomy. World J Urol 33(11):1801–1806
Zheng Y, Espiritu P, Hakky T, Jutras K, Spiess PE (2014) Predicting ease of perinephric fat dissection at time of open partial nephrectomy using preoperative fat density characteristics: preoperative fat characteristics for predicting ease of dissection at OPN. BJU Int 114(6):872–880
Davidiuk AJ, Parker AS, Thomas CS, Leibovich BC, Castle EP, Heckman MG et al (2014) Mayo adhesive probability score: an accurate image-based scoring system to predict adherent perinephric fat in partial nephrectomy. Eur Urol 66(6):1165–1171
Gillies RJ, Kinahan PE, Hricak H (2016) Radiomics: images are more than pictures, they are data. Radiology 278(2):563–577
Materka A, Strzelecki M et al (1998) Texture analysis methods—a review. Technical University of Lodz, Institute of Electronics, COST B11 report, Brussels, pp 9–11
Simpson AL, Adams LB, Allen PJ, D’Angelica MI, DeMatteo RP, Fong Y et al (2015) Texture analysis of preoperative CT images for prediction of postoperative hepatic insufficiency: a preliminary study. J Am Coll Surg 220(3):339–346
Ryu YJ, Choi SH, Park SJ, Yun TJ, Kim J-H, Glioma Sohn C-H (2014) Application of Whole-tumor texture analysis of diffusion-weighted imaging for the evaluation of tumor heterogeneity. PLoS ONE. 9(9):e108335
Canellas R, Mehrkhani F, Patino M, Kambadakone A, Sahani D (2016) Characterization of portal vein thrombosis (neoplastic versus bland) on CT images using software-based texture analysis and thrombus density (hounsfield units). Am J Roentgenol 207(5):W81–W87
Gnep K, Fargeas A, Gutiérrez-Carvajal RE, Commandeur F, Mathieu R, Ospina JD et al (2017) Haralick textural features on T 2-weighted MRI are associated with biochemical recurrence following radiotherapy for peripheral zone prostate cancer: impact of MRI in prostate cancer. J Magn Reson Imaging 45(1):103–117
Bylund JR, Qiong H, Crispen PL, Venkatesh R, Strup SE (2013) Association of clinical and radiographic features with perinephric ‘sticky’ fat. J Endourol 27(3):370–373
Davidiuk AJ, Parker AS, Thomas CS, Heckman MG, Custer K, Thiel DD (2015) Prospective evaluation of the association of adherent perinephric fat with perioperative outcomes of robotic-assisted partial nephrectomy. Urology 85(4):836–842
Collewet G, Strzelecki M, Mariette F (2004) Influence of MRI acquisition protocols and image intensity normalization methods on texture classification. Magn Reson Imaging 22(1):81–91
Szczypiński PM, Strzelecki M, Materka A, Klepaczko A (2009) MaZda—a software package for image texture analysis. Comput Methods Programs Biomed 94(1):66–76
Gibbs P, Turnbull LW (2003) Textural analysis of contrast-enhanced MR images of the breast. Magn Reson Med 50(1):92–98
Haralick RM (1979) Statistical and structural approaches to texture. Proc IEEE 67(5):786–804
Raman SP, Chen Y, Schroeder JL, Huang P, Fishman EK (2014) CT texture analysis of renal masses: pilot study using random forest classification for prediction of pathology. Acad Radiol 21(12):1587–1596
Sasaguri K, Takahashi N, Gomez-Cardona D, Leng S, Schmit GD, Carter RE et al (2015) Small (< 4 cm) renal mass: differentiation of oncocytoma from renal cell carcinoma on biphasic contrast-enhanced CT. AJR Am J Roentgenol 205(5):999–1007
Goh V, Ganeshan B, Nathan P, Juttla JK, Vinayan A, Miles KA (2011) Assessment of response to tyrosine kinase inhibitors in metastatic renal cell cancer: CT texture as a predictive biomarker. Radiology 261(1):165–171
Macleod LC, Hsi RS, Gore JL, Wright JL, Harper JD (2014) Perinephric fat thickness is an independent predictor of operative complexity during robot-assisted partial nephrectomy. J Endourol 28(5):587–591
Dariane C, Le Guilchet T, Hurel S, Audenet F, Beaugerie A, Badoual C et al (2017) Prospective assessment and histological analysis of adherent perinephric fat in partial nephrectomies. Urol Oncol Semin Orig Investig 35(2):39.e9–39.e17
Lubner MG, Stabo N, Lubner SJ, del Rio AM, Song C, Halberg RB et al (2015) CT textural analysis of hepatic metastatic colorectal cancer: pre-treatment tumor heterogeneity correlates with pathology and clinical outcomes. Abdom Imaging 40(7):2331–2337
Acknowledgements
Z.-E. Khene would like to thank the French Young Urological Association for his funding.
Author information
Authors and Affiliations
Contributions
Z-EK, KB, AL, SS, GV, BP, OA, RD, RM: Project development. Z-EK, AL, RM, GV, BP: Data collection. Z-EK, KB, RM, SS, OA, RD: Data analysis. Z-EK, KB, AL, SS, GV, BP, OA, RD, RM: Manuscript editing.
Corresponding author
Ethics declarations
Conflict of interest
Karim Bensalah and Gregory Verhoest are consultants for Intuitive Surgical.
Ethical standard
Local ethics committee approval.
Informed consent
Informed consent was obtained from all individual participants included in the study. This study and all the related procedures have been performed in accordance with the Declaration of Helsinki.
Rights and permissions
About this article
Cite this article
Khene, Z., Bensalah, K., Largent, A. et al. Role of quantitative computed tomography texture analysis in the prediction of adherent perinephric fat. World J Urol 36, 1635–1642 (2018). https://doi.org/10.1007/s00345-018-2292-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-018-2292-9