Abstract
Introduction
Evidence for sequencing targeted therapy (TT) in patients with metastatic renal cell carcinoma (mRCC) beyond third line is limited. Treatment decisions for these sequence options are largely based on individual preferences and experience. The aim of this study was to describe the efficacy and toxicity of fourth-line TT.
Materials and methods
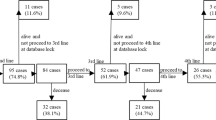
We retrospectively reviewed patients treated with fourth-line TT for mRCC after failure of previous treatment lines at a German academic high-volume center. Out of 406 patients treated in first line, 56 patients (14.8 %) were identified with more than three lines of TT. Progression-free survival (PFS) and overall survival (OS) were estimated using the Kaplan–Meier method. Cox proportional hazards models were applied to explore predictors of PFS and OS in uni- and multivariable analysis.
Results
For the fourth-line treatment, disease control rate was 35.7 %. Median OS from beginning of first-line therapy was 47.4 months (IQR 31.0–76.5). Primary resistance at first-line TT, metastatic disease at initial diagnosis and an intermediate MSKCC score were independent predictors of shorter OS from start of first-line TT. Median OS from the time of initiation of fourth-line therapy was 10.5 months (IQR 5.6–22.6). The corresponding median PFS for fourth-line TT was 3.2 months (IQR 1.6–8.0) and was not correlated with treatment response in first-line TT. The rate of toxicity-induced treatment termination was 16.1 %. Limitations are the retrospective and unicentric design with a limited number of patients.
Conclusions
Patients might benefit from subsequent treatment lines independently from treatment response in first line.


Similar content being viewed by others
References
Mills EJ et al (2009) Metastatic renal cell cancer treatments: an indirect comparison meta-analysis. BMC Cancer 9:34
Coon Thompson (2009) Sunitinib and bevacizumab for first-line treatment of metastatic renal cell carcinoma: a systematic review and indirect comparison of clinical effectiveness. Br J Cancer 101(2):238–243
Sternberg CN et al (2013) A randomised, double-blind phase III study of pazopanib in patients with advanced and/or metastatic renal cell carcinoma: final overall survival results and safety update. Eur J Cancer 49(6):1287–1296
Motzer RJ et al (2013) Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N Engl J Med 369(8):722–731
Hudes G et al (2007) Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med 356(22):2271–2281
Motzer RJ et al (2013) Axitinib versus sorafenib as second-line treatment for advanced renal cell carcinoma: overall survival analysis and updated results from a randomised phase 3 trial. Lancet Oncol 14(6):552–562
Rini BI et al (2011) Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial. Lancet 378(9807):1931–1939
Motzer RJ et al (2008) Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet 372(9637):449–456
Escudier B et al (2007) Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med 356(2):125–134
Ljungberg B et al (2015) EAU guidelines on renal cell carcinoma: 2014 update. Eur Urol 67(5):913–924
Gruenwald V et al (2014) ASET study: long-term follow-up data of patients with metastatic renal cell carcinoma treated with MGN1601. J Clin Oncol 32(suppl 4; abstr LBA399)
Grünwald V et al (2014) 1063PASET study: final results of patients with locally recurrent or metastatic renal cell carcinoma (RCC) treated with MGN1601. Ann Oncol 25(Suppl 4):iv366–iv367
Therasse P et al (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92(3):205–216
Trotti A et al (2003) CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol 13(3):176–181
Busch J et al (2013) Retrospective comparison of triple-sequence therapies in metastatic renal cell carcinoma. Eur Urol 64(1):62–70
Iacovelli R et al (2013) Clinical outcomes in patients receiving three lines of targeted therapy for metastatic renal cell carcinoma: results from a large patient cohort. Eur J Cancer 49(9):2134–2142
Ko JJ et al (2014) First-, second-, third-line therapy for mRCC: benchmarks for trial design from the IMDC. Br J Cancer 110(8):1917–1922
Vallet S et al (2015) Efficacy of targeted treatment beyond third-line therapy in metastatic kidney cancer: retrospective analysis from a large-volume cancer center. Clin Genitourin Cancer 13(3):e145–e152
Maute L et al (2014) Therapy of mRCC beyond mTOR-inhibition in clinical practice: results of a retrospective analysis. J Cancer Res Clin Oncol 140(5):823–827
Motzer RJ et al (2014) Dovitinib versus sorafenib for third-line targeted treatment of patients with metastatic renal cell carcinoma: an open-label, randomised phase 3 trial. Lancet Oncol 15(3):286–296
Calvo E et al (2012) Everolimus in metastatic renal cell carcinoma: subgroup analysis of patients with 1 or 2 previous vascular endothelial growth factor receptor-tyrosine kinase inhibitor therapies enrolled in the phase III RECORD-1 study. Eur J Cancer 48(3):333–339
Busch J et al (2011) Intrinsic resistance to tyrosine kinase inhibitors is associated with poor clinical outcome in metastatic renal cell carcinoma. BMC Cancer 11:295
Heng DY et al (2012) Primary anti-vascular endothelial growth factor (VEGF)-refractory metastatic renal cell carcinoma: clinical characteristics, risk factors, and subsequent therapy. Ann Oncol 23(6):1549–1555
Heng D Y et al (2015) Third-line therapy in metastatic renal cell carcinoma: results from the International mRCC Database Consortium. J Clin Oncol 33(7; abstr 430)
Heng DY et al (2009) Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: results from a large, multicenter study. J Clin Oncol 27(34):5794–5799
Acknowledgments
B Ralla is a participant in the Charité—Junior Clinical Scientist Program funded by the Charité—Universitaetsmedizin Berlin and the Berlin Institute of Health. J Busch is a participant in the Charité—Twinning Grant Program funded by the Charité—Universitaetsmedizin Berlin and the Berlin Institute of Health.
Author contributions
B Ralla contributed to data collection, data analysis and manuscript writing; J Busch and C Kempkensteffen analyzed the data and edited the manuscript; K Miller, A Magheli and S Hinz edited the manuscript; B Erber collected the data; A Flörcken collected the data and edited the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
B Ralla and L von der Aue: none; J Busch: advisor to Pfizer, Novartis, GSK, Roche, Mologen; K Miller: advisor to Pfizer, Novartis, Roche, BMS, MSD; A Magheli advisor to Pfizer; B Erber: advisor to Pfizer, Novartis, GSK, Roche; C Kempkensteffen advisor to Astellas, Jansen-Cilag, Bayer, Pfizer; S Hinz: advisor to Pfizer and Novartis; A Flörcken: advisor to Bayer, Pfizer; I Goranova: advisor to Novartis.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
The study was approved by the Charité – University Hospital Ethics Committee. Informed consent was obtained from the participants.
Rights and permissions
About this article
Cite this article
Ralla, B., Erber, B., Goranova, I. et al. Efficacy of fourth-line targeted therapy in patients with metastatic renal cell carcinoma: a retrospective analysis. World J Urol 34, 1147–1154 (2016). https://doi.org/10.1007/s00345-015-1740-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-015-1740-z