Abstract
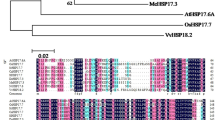
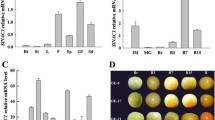
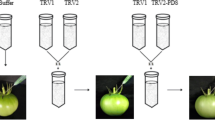
The productivity of many horticultural crops is determined by the fruit set. A complex hormone network regulates fruit setting, but the involvement of abscisic acid (ABA) in this regulation is unknown. SlNCED1, which encodes 9-cis-epoxycarotenoid dioxygenase (NCED), a crucial enzyme in ABA production, was studied using RNA interference (RNAi) controlled by a fruit-specific E8 promoter to establish the significance of ABA in fruit setting. The wild-type (WT) tomato pistil's endogenous ABA content and SlNCED1 expression increased from six days before full bloom (− 6 DAF, days after full bloom) to the day of full bloom (0 DAF), and peaked at the − 2 DAF and − 1 DAF, respectively. The content of endogenous ABA and the expression level of SlNCED1 decreased gradually from 0 to 6 DAF. The expression of SlNCED1 in the pistil of transgenic tomato decreased by 40.2%–63.4% compared with WT. The content of ABA in the pistil of transgenic tomato decreased by 35.3%–73.3% compared with WT. In addition, SlNCED1-RNAi considerably reduced the expression of ABA signaling pathway genes SlPYL3 (ABA receptor), SlPP2C1/2/5 (type 2C protein phosphatase), and SnRK2.6 (subfamily 2 of SNF1-related kinases), which strongly repress the ABA signaling transduction. These changes resulted in the aberrant development of the pistil. The fruit setting rate of tomatoes declined drastically to 9.1%. All fruits were parthenocarpic with a considerable proportion of fruit of abnormal shape. In conclusion, the disruption of endogenous ABA balance caused by fruit-specific SlNCED1-RNAi affects tomato pistil development and fruit set.








Similar content being viewed by others
References
Ariizumi T, Amagai M, Shibata D, Hatakeyama K, Watanabe M (2002) Comparative study of promoter activity of three anther-specific genes encoding lipid transfer protein, xyloglucan endotransglucosylase/hydrolase and polygalacturonase in transgenic Arabidopsis thaliana. Plant Cell Rep 21:90–96
Azzi L, Deluche C, Gevaudant F, Frangne N, Delmas F, Hernould M, Chevalier C (2015) Fruit growth-related genes in tomato. J Exp Bot 66:1075–1086
Chandra Sekhar KN, Sawhney VK (1991) Role of ABA in stamen and pistil development in the normal and solanifolia mutant of tomato (Lycopersicon esculentum). Sex Plant Reprod 4:279–283
Cong L, Wu T, Liu H, Wang H, Zhang H, Zhao G, Wen Y, Shi Q, Xu L, Wang Z (2020) CPPU may induce gibberellin-independent parthenocarpy associated with PbRR9 in ‘Dangshansu’pear. Hortic Res 7:1–13
Dai S, Kai W, Liang B, Wang J, Jiang L, Du Y, Sun Y, Leng P (2018) The functional analysis of SlNCED1 in tomato pollen development. Cell Mol Life Sci 75:3457–3472
Di C, Yuan J, Wu Y, Li J, Lin H, Hu L, Zhang T, Qi Y, Gerstein M, Guo Y, Lu ZJ (2014) Characterization of stress-responsive lncRNAs in Arabidopsis thaliana by integrating expression, epigenetic and structural features. Plant J 80:848–861
Dorcey E, Urbez C, Blázquez MA, Carbonell J, Perez-Amador MA (2009) Fertilization-dependent auxin response in ovules triggers fruit development through the modulation of gibberellin metabolism in Arabidopsis. Plant J 58:318–332
Ezura K, Ji-Seong K, Mori K, Suzuki Y, Kuhara S, Ariizumi T, Ezura H (2017) Genome-wide identification of pistil-specific genes expressed during fruit set initiation in tomato (Solanum lycopersicum). PLoS ONE 12:e0180003–e0180037
Fenn MA, Giovannoni JJ (2020) Phytohormones in fruit development and maturation. Plant J 105:446–458
Fujii H, Chinnusamy V, Rodrigues A, Rubio S, Antoni R, Park S, Cutler SR, Sheen J, Rodriguez PL, Zhu J (2009) In vitro reconstitution of an abscisic acid signalling pathway. Nature 462:660–664
Gillaspy G, Ben-Davi H, Gruissem W (1993) Fruits: a developmental perspective. Plant Cell 5:1439–1451
Ji K, Chen P, Sun L, Wang Y, Dai S, Li Q, Li P, Sun Y, Wu Y, Duan C, Leng P (2012) Non-climacteric ripening in strawberry fruit is linked to ABA, FaNCED2 and FaCYP707A1. Funct Plant Biol 39:351–357
Ji K, Kai W, Zhao B, Sun Y, Yuan B, Dai S, Li Q, Chen P, Wang Y, Pei Y, Wang H, Guo Y, Leng P (2014) SlNCED1 and SlCYP707A2: key genes involved in ABA metabolism during tomato fruit ripening. J Exp Bot 65:5243–5255
Jia HF, Chai YM, Li CL, Lu D, Luo JJ, Qin L, Shen YY (2011) Abscisic acid plays an important role in the regulation of strawberry fruit ripening. Plant Physiol 157:188–199
Ju C, Van De Poel B, Cooper ED, Thierer JH, Gibbons T, Delwiche CF, Chang C (2015) Conservation of ethylene as a plant hormone over 450 million years of evolution. Nature Plants 1:14004–14010
Kai W, Fu Y, Wang J, Liang B, Li Q, Leng P (2019) Functional analysis of SlNCED1 in pistil development and fruit set in tomato (Solanum lycopersicum L.). Sci Rep 9:16943–16955
Kim G, LeBlanc ML, Wafula EK, de Pamphilis C, Westwood JH (2014) Genomic-scale exchange of mRNA between a parasitic plant and its hosts. Science 345:808–811
Kim J-S, Ezura K, Lee J, Kojima M, Takebayashi Y, Sakakibara H, Ariizumi T, Ezura H (2020) The inhibition of SlIAA9 mimics an increase in endogenous auxin and mediates changes in auxin and gibberellin signalling during parthenocarpic fruit development in tomato. J Plant Physiol 252:153238
Koenig D, Jimenezgomez JM, Kimura S, Fulop D, Chitwood DH, Headland LR, Kumar R, Covington MF, Devisetty UK, Tat AV, Tohge T, Bolger A, Schneeberger K, Ossowski S, Lanz C, Xiong G, Taylorteeples M, Brady SM, Pauly M, Weigel D, Usadel B, Fernie AR, Peng J, Sinha N, Maloof JN (2013) Comparative transcriptomics reveals patterns of selection in domesticated and wild tomato. Proc Natl Acad Sci USA 110:E2655–E2662
Liao X, Li M, Liu B, Yan M, Yu X, Zi H, Liu R, Yamamuro C (2018) Interlinked regulatory loops of ABA catabolism and biosynthesis coordinate fruit growth and ripening in woodland strawberry. Proc Natl Acad Sci USA 115:e11542–e11550
Matsuo S, Miyatake K, Endo M, Urashimo S, Fukuoka H (2020) Loss of function of the Pad-1 aminotransferase gene, which is involved in auxin homeostasis, induces parthenocarpy in Solanaceae plants. Proc Natl Acad Sci USA 117:12784–12790
Mesejo C, Yuste R, Reig C, Martínez-Fuentes A, Iglesias DJ, Muñoz-Fambuena N, Bermejo A, Germanà MA, Primo-Millo E, Agustí M (2016) Gibberellin reactivates and maintains ovary-wall cell division causing fruit set in parthenocarpic Citrus species. Plant Sci 247:13–24
Mezzetti B, Landi L, Pandolfini T, Spena A (2004) The defH9-iaaM auxin-synthesizing gene increases plant fecundity and fruit production in strawberry and raspberry. BMC Biotechnol 4:4–13
Nitsch LMC, Oplaat C, Feron R, Ma Q, Wolters-Arts M, Hedden P, Mariani C, Vriezen WH (2009) Abscisic acid levels in tomato ovaries are regulated by LeNCED1 and SlCYP707A1. Planta 229:1335–1346
Orourke JA, Yang SS, Miller SS, Bucciarelli B, Liu J, Rydeen A, Bozsoki Z, Uhdestone C, Tu ZJ, Allan DL, Gronwald JW, Vance CP (2013) An RNA-Seq transcriptome analysis of orthophosphate-deficient white lupin reveals novel insights into phosphorus acclimation in plants. Plant Physiol 161:705–724
Park S, Fung P, Nishimura N, Jensen DR, Fujii H, Zhao Y, Lumba S, Santiago J, Rodrigues A, Chow TF, Alfred SE, Bonetta D, Finkelstein R, Provart NJ, Desveaux D, Rodriguez PL, McCourt P, Zhu JK, Schroeder JI, Volkman BF, Cutler SR (2009) Abscisic acid inhibits type 2C protein phosphatases via the PYR/PYL family of START proteins. Science 324:1068–1071
Pattison RJ, Csukasi F, Zheng Y, Fei Z, van der Knaap E, Catalá C (2015) Comprehensive tissue-specific transcriptome analysis reveals distinct regulatory programs during early tomato fruit development. Plant Physiol 168:1684–1701
Pesares P, Mizzotti C, Colombo M, Masiero S (2014) Genetic regulation and structural changes during tomato fruit development and ripening. Front Plant Sci 5:124–137
Ruan YL, Patrick JW, Bouzayen M, Osorio S, Fernie AR (2012) Molecular regulation of seed and fruit set. Trends Plant Sci 17:656–665
Ruiu F, Picarella ME, Imanishi S, Mazzucato A (2015) A transcriptomic approach to identify regulatory genes involved in fruit set of wild-type and parthenocarpic tomato genotypes. Plant Mol Biol 89:263–278
Serrani JC, Fos M, Atares A, Garcia-Martinez JL (2007) Effect of gibberellin and auxin on parthenocarpic fruit growth induction in the cv Micro-Tom of tomato. J Plant Growth Regul 26:211–221
Serrani JC, Ruiz-Rivero O, Fos M, Garcia-Martinez JL (2008) Auxin-induced fruit-set in tomato is mediated in part by gibberellins. Plant J 56:922–934
Sharif R, Su L, Chen X, Qi X (2022) Hormonal interactions underlying parthenocarpic fruit formation in horticultural crops. Horticult Res. https://doi.org/10.1093/hr/uhab024
Shen X, Wang Z, Song X, Xu J, Jiang C, Zhao Y, Ma C, Zhang H (2014) Transcriptomic profiling revealed an important role of cell wall remodeling and ethylene signaling pathway during salt acclimation in Arabidopsis. Plant Mol Biol 86:303–317
Sotelo-Silveira M, Marsch-Martínez N, de Folter S (2014) Unraveling the signal scenario of fruit set. Planta 239:1147–1158
Sun L, Zhang M, Ren J, Qi J, Zhang G, Leng P (2010) Reciprocity between abscisic acid and ethylene at the onset of berry ripening and after harvest. BMC Plant Biol 10:257–267
Sun L, Wang Y, Chen P, Ren J, Ji K, Li Q, Li P, Dai S, Leng P (2011) Transcriptional regulation of SlPYL, SlPP2C, and SlSnRK2 gene families encoding ABA signal core components during tomato fruit development and drought stress. J Exp Bot 62:5659–5669
Sun L, Sun Y, Zhang M, Wang L, Ren J, Cui M, Wang Y, Ji K, Li P, Li Q, Chen P, Dai S, Duan C, Wu Y, Leng P (2012) Suppression of 9-cis-epoxycarotenoid dioxygenase, which encodes a key enzyme in abscisic acid biosynthesis, alters fruit texture in transgenic tomato. Plant Physiol 158:283–298
Tan S, Luschnig C, Friml J (2021) Pho-view of Auxin: reversible protein phosphorylation in auxin biosynthesis, transport and signalling. Mol Plant 14:151–165
Tang N, Den W, Hu G, Hu N, Li Z (2015) Transcriptome profiling reveals the regulatory mechanism underlying pollination dependent and parthenocarpic fruit set mainly mediated by auxin and gibberellin. PLoS ONE 10:e0125355
Umezawa T, Sugiyama N, Mizoguchi M, Hayashi S, Myouga F, Yamaguchi-Shinozaki K, Ishihama Y, Hirayama T, Shinozaki K (2009) Type 2C protein phosphatases directly regulate abscisic acid-activated protein kinases in Arabidopsis. Proc Natl Acad Sci USA 106:17588–17593
Voß U, Wilson MH, Kenobi K, Gould PD, Robertson FC, Peer WA, Lucas M, Swarup K, Casimiro I, Holman TJ, Wells DM, Péret B, Goh T, Fukaki H, Hodgman TC, Laplaze L, Halliday KJ, Ljung K, Murphy AS, Hall AJ, Webb AAR, Bennetta MJ (2015) The circadian clock rephrases during lateral root organ initiation in Arabidopsis thaliana. Nat Commun 6:7641–7649
Vriezen WH, Feron R, Maretto F, Keijman J, Mariani C (2008) Changes in tomato ovary transcriptome demonstrate complex hormonal regulation of fruit set. New Phytol 177:60–76
Weng L, Zhao F, Li R, Xu C, Chen K, Xiao H (2015) The zinc finger transcription factor SlZFP2 negatively regulates abscisic acid biosynthesis and fruit ripening in tomato. Plant Physiol 167:931–949
Xiao HS, Lv LX, Chen ZT (2003) Dynamic changes of endogenous hormone in litchi (Litchi chinensis sonn.) pistil and stamen during flower development. J Environ Sci 9:279–283
Zhou J, Sittmann J, Guo L, Xiao Y, Liu Z (2021) Gibberellin and auxin signaling genes RGA1 and ARF8 repress accessory fruit initiation in diploid strawberry. Physiol Plant 185:1059–1075
Zou J, Abrams GD, Barton DL, Taylor DC, Pomeroy MK, Abrams SR (1995) Induction of lipid and oleosin biosynthesis by (+)-abscisic acid and its metabolites in microspore-derived embryos of Brassica napus L. cv Reston. Plant Physiol 108:563–571
Funding
This research was financially supported by grants from the National Natural Science Foundation of China (Grant No. 31902018, 32070344) and Natural Science Foundation of Shandong Province (Grant No. ZR2019PC016).
Author information
Authors and Affiliations
Contributions
SD and ZL: conceptual and experiment designs; XW, XC and SS: experiments were conducted; XW, MX and SD: data analysis performed; XZ, HW and XZ: reagents/materials/analysis tools were contributed. SD and ZL: The report was written. All the authors have commented, read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling Author: Abdul Latif Khan.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, X., Cui, X., Shang, S. et al. The Destruction of Endogenous ABA Balance Caused by Fruit-specific SlNCED1-RNAi Affects Tomato Fruit Set. J Plant Growth Regul 42, 7200–7214 (2023). https://doi.org/10.1007/s00344-023-11008-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-023-11008-w