Abstract
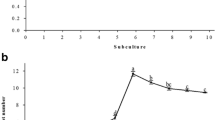
Citrus fruits are the most consumed fruit in the world and Mexico is the first producer of Persian lime. Prized for their larger size, less intense acidity, and absence of seeds, the fruits are used both for fresh consumption and industrial use. However, they present a number of phytosanitary problems and there is a great demand for disease-free plants. The present work aims to identify an in vitro culture system for the propagation of Persian lime, which allows obtaining of healthy and vigorous clones and ensures their genetic stability in subcultures. The effect of three in vitro culture systems and subcultures on the somaclonal variation of Persian lime (Citrus × latifolia) was analyzed using ISSR (inter simple sequence repeat) markers. Shoots of 0.5 cm in length were formed and multiplied in three in vitro culture systems in Murashige and Skoog medium supplemented with 1.0 mg L−1 6-benzylaminopurine and 0.5 mg L−1 kinetin. After 30 days, the number and length of each axillary shoot, as well as the concentration of phytohormones, were quantified. The RITA® system had the highest number of 3.8 shoots in the third subculture and the lowest somaclonal variation of less than 10.24%, with an increase in the concentration of phytohormones 6-benzylaminopurine and kinetin. The semisolid culture system had the highest percentage of variation of 26.5% in the eighth subculture.





Similar content being viewed by others
Data Availability
Not applicable.
Code Availability
Not applicable.
References
Agisimanto D, Normah MN, Ibrahim R (2019) Rapid somatic embryogenesis of Citrus reticulata Blanco cv. Madu in an air-lift bioreactor culture. Agrivita 41(2):284–294
Albany de Vilchez NR, Vilchez JA, León de Sierralta S, Nava Fereira AR, Martínez Ferrer LJ, Molina MA (2015) Liquid medium culture: an approach for the commercial micropropagation of aloe (Aloe barbadensis Mill). Rev Colomb Biotecnol 17(1):24–31
Azizi P, Hanafi MM, Sahebi M, Harikrishna JA, Taheri S, Yassoralipour A, Nasehi A (2020) Epigenetic changes and their relationship to somaclonal variation: a need to monitor the micropropagation of plantation crops. Funct Plant Biol 47(6):508–523
Bairu MW, Fennell CW, van Staden J (2006) The effect of plant growth regulators on somaclonal variation in Cavendish banana (Musa AAA cv. ‘Zelig’). Sci Hortic 108:347–351
Bairu MW, Aremu AO, Van Staden J (2011) Somaclonal variation in plants: causes and detection methods. Plant Growth Regul 63(2):147–173
Bulbarela-Marini JE, Gómez-Merino F, Galindo-Tovar ME, Solano-Rodríguez LA, Murguía-González J, Pastelín-Solano MC, Nuñez-Pastrana R, Castañeda-Castro O (2019) The in vitro propagation system of Citrus × latifolia (Yu. Tanaka) Yu. Tanaka (Rutaceae) affects the growth and depletion of nutriments. In Vitro Cell Dev Biol Plant 55:290–295
Cengiz M, Kaçar YA (2019) Micropropagation of some citrus rootstocks with classical and new generation tissue culture techniques. TURJAF 7(9):1469–1478
Coenen C, Christian M, Lüthen H, Lomax TL (2003) Cytokinin inhibits a subset of diageotropica-dependent primary auxin responses in tomato. Plant Physiol 131(4):1692–1704
Chambers GK, MacAvoy ES (2000) Microsatellites: consensus and controversy. Comp Biochem Physiol 126:455–476
Doyle JJ, Doyle JL (1990) Isolation of plant DNA from fresh tissue. Focus 12:13–15
El-Sherif NA (2018) Impact of plant tissue culture on agricultural sustainability. In: Sustainability of agricultural environment in Egypt: Part II. Springer, Cham, pp 93–107
Fang D, Krueger RR, Roose ML (1998) Phylogenetic relationships among selected Citrus germplasm accessions revealed by inter-simple sequence repeat (ISSR) markers. J Am Soc Hortic Sci 123(4):612–617
FAOSTAT (2018) Producción de limones y limas. www.fao.org/faostat. Accessed 2 November 2020
Fernández G, Aguilar A, Azzaro-Pantel C, Miranda-Ackerman M, Purroy R (2015) Behavior patterns related to the agricultural practices in the production of Persian lime (Citrus latifolia tanaka) in the seasonal orchard. Comput Electron Agric 116:162–172
Georgieva L, Tsvetkov I, Georgieva M, Kondakova V (2016) New protocol for in vitro propagation of berry plants by TIS bioreactor. Bulg J Agric Sci 22:745–751
Hao Y, Deng X (2002) Occurrence of chromosomal variations and plant regeneration from long-term-cultured citrus callus. In Vitro Cell Dev Biol Plant 38:472–476
Haradzi NA, Khor SP, Subramaniam S, Chew BL (2021) Regeneration and micropropagation of Meyer lemon (Citrus x meyeri) supported by polymorphism analysis via molecular markers. Sci Hortic 286:110225
Hassanzadeh H, Rastegar S, Golein B, Golmohammadi M, Aboutalebi A (2017) Genetic diversity in Persian lime (Citrus latifolia tanaka) accessions using morphological and molecular markers. Agric for 63:221–231
Kamvar ZN, Tabima JF, Grünwald NJ (2014) Poppr: an R package for genetic analysis of populations with clonal, partially clonal, and/or sexual reproduction. PeerJ 2:e281
Kaur M, Dhaliwal HS, Thakur A, Singh G, Kaur M (2015) In vitro plantlet formation in Carrizo citrange: a promising citrus rootstock. Indian J Hortic 72(1):1–6
Kassambara A (2017) Practical guide to cluster analysis in R: unsupervised machine learning, vol 1. STHDA, London, pp 44–57
Kim HJ, Lee JN, Choi MJ, Suh JT (2019) Comparison of in vitro propagation and occurrence of morphological and genetic variation in strawberry tissue culture with various plant hormone treatments. J Plant Biotechnol 46(2):106–113
Krishna H, Alizadeh M, Singh D, Singh U, Chauhan N, Eftekhari M, Sadh RK (2016) Somaclonal variations and their applications in horticultural crops improvement. 3 Biotech 6(1):54
Kritskaya TA, Kashin AS, Kasatkin MY (2019) Micropropagation and somaclonal variation of Tulipa suaveolens (Liliaceae) in vitro. Russ J Dev Biol 50:209–215
Kılınç FM, Süzerer V, Çiftçi YÖ, Onay A, Yıldırım H, Uncuoğlu AA, Tilkat E, Koc I, Akdemir OF, Metin ÖK (2015) Clonal micropropagation of Pistacia lentiscus L. and assessment of genetic stability using IRAP markers. Plant Growth Regul 75:75–88
Martínez-Estrada E, Caamal-Velázquez JH, Salinas-Ruíz J, Bello-Bello JJ (2017) Assessment of somaclonal variation during sugarcane micropropagation in temporary immersion bioreactors by intersimple sequence repeat (ISSR) markers. In Vitro Cell Dev Biol Plant 53(6):553–560
Mosqueda-Frómeta O, Escalona-Morgado MM, Teixeira-da-Silva JA, Pina-Morgado DT, Daquinta-Gradaille MA (2017) In vitro propagation of Gerbera jamesonii Bolus ex Hooker f. in a temporary immersion bioreactor. Plant Cell Tiss Organ Cult 129(3):543–551
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15(3):473–497
Pastelín-Solano MC, Salinas-Ruíz J, González-Arnao MT, Castañeda-Castro O, Galindo-Tovar ME, Bello-Bello JJ (2019) Evaluation of in vitro shoot multiplication and ISSR marker based assessment of somaclonal variants at different subcultures of vanilla (Vanilla planifolia Jacks). Physiol Mol Biol Plants 25:561–567
Pérez LP, Montesinos YP, Olmedo JG, Sánchez RR, Montenegro ON, Rodríguez RB, Ribalta OH, Escriba RCR, Daniels D, Gómez-Kosky R (2015) Effects of different culture conditions (photoautotrophic, photomixotrophic) and the auxin indole-butyric acid on the in vitro acclimatization of papaya (Carica papaya L. var. Red Maradol) plants using zeolite as support. Afr J Biotechnol 14(35):2622–2635
Prakash S, Sharma S (2018) Micropropagation of citrus rootstock. J Pharmacogn Phytochem 7(4):1513–1517
Salis C, Papadakis IE, Kintzios S, Hagidimitriou M (2017) In vitro propagation and assessment of genetic relationships of citrus rootstocks using ISSR molecular markers. Not Bot Horti Agrobot Cluj Napoca 45(2):383–391
Savita PPK, Virk GS, Nagpal A (2015) An efficient somatic embryogenesis protocol for Citrus jambhiri and assessment of clonal fidelity of plantlets using RAPD markers. J Plant Growth Regul 34(2):309–319
Shashikumar R, Krishna V, Lokesha S (2017) Analysis of genetic variations in Musa paradisiaca cv Karibale-Monthan using AFLP markers. Indian J Agric Sci 51(6):543–549
SIAP (2018) Avance de siembras y cosechas Resumen nacional por cultivo. http://infosiap.siap.gob.mx/. Accessed 2 November 2020
Soares DMM, Sattler MC, da Silva Ferreira MF, Praça-Fontes MM (2016) Assessment of genetic stability in three generations of in vitro propagated Jatropha curcas L. plantlets using ISSR markers. Trop Plant Biol 9:229–238
Thomas J, Vijayan D, Joshi SD, Lopez SJ, Kumar RR (2006) Genetic integrity of somaclonal variants in tea (Camellia sinensis (L.) O Kuntze) as revealed by inter simple sequence repeats. J Biotechnol 123(2):149–154
Valdiani A, Hansen O, Nielsen U, Johannsen V, Shariat M, Georgiev M, Omidvar V, Ebrahimi M, Dinanai E, Abiri R (2019) Bioreactor-based advances in plant tissue and cell culture: challenges and prospects. Crit Rev Biotechnol 39(1):20–34
Wang N (2019) The citrus huanglongbing crisis and potential solutions. Mol Plant 12(5):607–609
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
Study design JEBM, FCGM, OCC. Conducting study JEBM, OCC. Writing and proofreading JEBM, OCC, MEGT, MCPS, RNP, JMG, JEBM data analysis.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Consent to Participate
The authors declare that they obtained an informed consent to participate from people involved in this study.
Ethical Approval
Not applicable.
Additional information
Handling Editor: Stefaan Werbrouck.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bulbarela-Marini, J.E., Gómez-Merino, F.C., Galindo-Tovar, M.E. et al. Ratio of Somaclonal Variation and the Phytohormonal Content of Citrus × latifolia in Three In Vitro Culture Systems. J Plant Growth Regul 42, 3356–3364 (2023). https://doi.org/10.1007/s00344-022-10796-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-022-10796-x