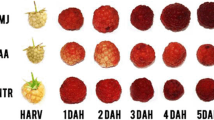
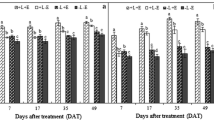
Abstract
Auxin is a hormone that delays ripening in part by reducing anthocyanin content and impairing color development. Auxin content declines during the ripening process, whereas sugars accumulate from pre-veraison onwards. The spatio-temporal distribution of this hormone depends in part on polar auxin transport. On the other hand, sugar, acting as a signal molecule, modulates auxin distribution in model organisms; however, its effect on polar auxin transport during the coloring process in grapevine berries has not been investigated. To address this issue, we characterized auxin transport and sugar variations during the ripening process and performed treatments intended to alter auxin homeostasis in grape fruits. We found that polar auxin transport declines in concert with increasing sugar content prior to and at veraison. Moreover, N-1-napthylphthalamic acid (NPA; a polar auxin transport inhibitor) and glucose treatment increased berry coloration, reduced polar auxin transport and VvPIN1 transcript abundance at pre-veraison, and combined NPA and glucose treatment further increased berry color compared to glucose and NPA alone. Indole-3-acetic acid (IAA) treatment prevented the negative effect of glucose on auxin transport, suggesting that auxin homeostasis might be relevant for glucose modulation of berry ripening in grapevine. Impaired auxin transport is associated with increased ethylene sensitivity in several plant processes, including fruit abscission. For exploring a potential involvement of the ethylene pathway during the coloring process, we analyzed the transcript abundance of the putative ethylene receptor, VvETR2, a possible negative regulator of the ethylene pathway. We found that glucose plus NPA treatment reduced VvETR2 transcript abundance, whose expression is reported to decline from veraison onwards. Our results suggest a possible mechanism in which a rise in glucose contributes to auxin transport inhibition in coloring berries. As glucose has been reported to promote ripening and IAA inhibits berry coloring, our results further support an antagonistic switch between IAA and glucose, that could also involve changes in the expression of the VvETR2 gene.





Similar content being viewed by others
Abbreviations
- NCED:
-
9-Cis-epoxy-carotenoid dioxygenase
- ABA:
-
Abscisic acid
- E-L:
-
Eichhorn-Lorenz
- ETR:
-
Ethylene receptor
- ERS:
-
Ethylene response sensor
- NPA:
-
N-1-napthylphthalamic acid
- PIN:
-
PIN-FORMED
References
Bangerth F (2000) Abscission and thinning of young fruit and thier regulation by plant hormones and bioregulators. Plant Growth Regul 31:43–59
Bao BL, Ke LQ, Jiang JM, Ying TJ (2007) Fruit quality of transgenic tomatoes with suppressed expression of LeETR1 and LeETR2 genes. Asia Pac J Clin Nutr 16:122–126
Böttcher C, Keyzers RA, Boss PK, Davies C (2010) Sequestration of auxin by the indole-3-acetic acid-amido synthetase GH3-1 in grape berry (Vitis vinifera L.) and the proposed role of auxin conjugation during ripening. J Exp Bot 61:3615–3625
Böttcher C, Harvey K, Forde C, Boss P, Davies C (2011) Auxin treatment of pre-veraison grape (Vitis vinifera L.) berries both delays ripening and increases the synchronicity of sugar accumulation. Aust J Grape Wine Res 17:1–8
Böttcher C, Burbidge CA, Boss PK, Davies C (2013) Interactions between ethylene and auxin are crucial to the control of grape (Vitis vinifera L.) berry ripening. BMC Plant Biol 13:222
Böttcher C, Harvey KE, Boss PK, Davies C (2013b) Ripening of grape berries can be advanced or delayed by reagents that either reduce or increase ethylene levels. Funct Plant Biol 40:566–581
Cao WH, Liu J, He XJ, Mu RL, Zhou HL, Chen SY, Zhang JS (2007) Modulation of ethylene responses affects plant salt-stress responses. Plant Physiol 143:707–719
Chervin C, El-Kereamy A, Roustan JP, Latché A, Lamon J, Bouzayen M (2004) Ethylene seems required for the berry development and ripening in grape, a non-climacteric fruit. Plant Sci 167:1301–1305
Coombe BG (1976) The development of fleshy fruits. Annu Rev Plant Physiol 27:207–228
Coombe BG (1992) Research on development and ripening of the grape berry. Am J Enol Vitic 43:101–110
Coombe BG (1995) Adoption of a system for identifying grapevine growth stages. Aust J Grape Wine Res 1:100–110
Coombe BG, McCarthy MG (2000) Dynamics of grape berry growth and physiology of ripening. Aust J Grape Wine Res 6:131–135
Davies C, Boss PK, Robinson SP (1997) Treatment of grape berries, a nonclimacteric fruit with a synthetic auxin, retards ripening and alters the expression of developmentally regulated genes. Plant Physiol 115:1155–1161
Dekkers BJ, Schuurmans JA, Smeekens SC (2004) Glucose delays seed germination in Arabidopsis thaliana. Planta 218:579–588
Deluc LG, Grimplet J, Wheatley MD, Tillett RL, Quilici DR, Osborne C, Schooley DA, Schlauch KA, Cushman JC, Cramer GR (2007) Transcriptomic and metabolite analyses of Cabernet Sauvignon grape berry development. BMC Genom 8:429
Downey MO, Harvey JS, Robinson SP (2003) Synthesis of flavonols and expression of flavonol synthase genes in the developing grape berries of Shiraz and Chardonnay (Vitis vinifera L.). Aust J Grape Wine Res 9:110–121
Else MA, Stankiewicz-Davies AP, Crisp CM, Atkinson CJ (2004) The role of polar auxin transport through pedicels of Prunus avium L. in relation to fruit development and retention. J Exp Bot 55:2099–2109
Fortes AM, Teixeira RT, Agudelo-Romero P (2015) Complex interplay of hormonal signals during grape berry ripening. Molecules 20:9326–9343
Friend A, Trought M, Creasy G (2009) The influence of seed weight on the development and growth of berries and live green ovaries in Vitis vinifera L. cvs. Pinot Noir and Cabernet Sauvignon. Aust J Grape Wine Res 15:166–174
Friml J, Palme K (2002) Polar auxin transport–old questions and new concepts? Plant Mol Biol 49:273–284
Gambetta GA, Matthews MA, Shaghasi TH, McElrone AJ, Castellarin SD (2010) Sugar and abscisic acid signaling orthologs are activated at the onset of ripening in grape. Planta 232:219–234
Gouthu S, Deluc LG (2015) Timing of ripening initiation in grape berries and its relationship to seed content and pericarp auxin levels. BMC Plant Biol 15:46
Hanson J, Hanssen M, Wiese A, Hendriks MM, Smeekens S (2008) The sucrose regulated transcription factor bZIP11 affects amino acid metabolism by regulating the expression of ASPARAGINE SYNTHETASE1 and PROLINE DEHYDROGENASE2. Plant J 53:935–949
Jia H, Li C, Chai Y, Xing Y, Shen Y (2013) Sucrose promotes strawberry fruit ripening by stimulation of abscisic acid biosynthesis. Pak J Bot 45:169–176
Jia H, Xie Z, Wang C, Shangguan L, Qian N, Cui M, Liu Z, Zheng T, Wang M, Fang J (2017) Abscisic acid, sucrose, and auxin coordinately regulate berry ripening process of the Fujiminori grape. Funct Integr Genomics 17:441–457
Kuhn N, Guan L, Dai ZW, Wu BH, Lauvergeat V, Gomès E, Li S-H, Godoy F, Arce-Johnson P, Delrot S (2013) Berry ripening: recently heard through the grapevine. J Exp Bot 65:4543–4559
Kuhn N, Abello C, Godoy F, Delrot S, Arce-Johnson P (2014) Differential behavior within a grapevine cluster: decreased ethylene-related gene expression dependent on auxin transport is correlated with low abscission of first developed berries. PloS One 9:e111258
Kuhn N, Serrano A, Abello C, Arce A, Espinoza C, Gouthu S, Deluc L, Arce-Johnson P (2016) Regulation of polar auxin transport in grapevine fruitlets (Vitis vinifera L.) and the proposed role of auxin homeostasis during fruit abscission. BMC Plant Biol 16:234
Li J, Yuan R (2008) NAA and ethylene regulate expression of genes related to ethylene biosynthesis, perception, and cell wall degradation during fruit abscission and ripening in ‘Delicious’ apples. J Plant Growth Regul 27:283
Liu X, Cohen JD, Gardner G (2011) Low-fluence red light increases the transport and biosynthesis of auxin. Plant Physiol 157:891–904
Meir S, Philosoph-Hadas S, Sundaresan S, Selvaraj KV, Burd S, Ophir R, Kochanek B, Reid MS, Jiang C-Z, Lers A (2010) Microarray analysis of the abscission-related transcriptome in the tomato flower abscission zone in response to auxin depletion. Plant Physiol 154:1929–1956
Mishra BS, Singh M, Aggrawal P, Laxmi A (2009) Glucose and auxin signaling interaction in controlling Arabidopsis thaliana seedlings root growth and development. PloS One 4:e4502
Mounet F, Moing A, Kowalczyk M, Rohrmann J, Petit J, Garcia V, Maucourt M, Yano K, Deborde C, Aoki K, Bergès H, Granell A, Fernie AR, Bellini C, Rothan C, Lemaire-Chamley M (2012) Down-regulation of a single auxin efflux transport protein in tomato induces precocious fruit development. J Exp Bot 63:4901–4917
Muday GK, Haworth P (1994) Tomato root growth, gravitropism, and lateral development: correlation with auxin transport. Plant Physiol Biochem 32:193–203
Nishio S, Moriguchi R, Ikeda H, Takahashi H, Takahashi H, Fujii N, Guilfoyle TJ, Kanahama K, Kanayama Y (2010) Expression analysis of the. auxin efflux carrier family in tomato fruit development. Planta 232:755–764
Paciorek T, Zažímalová E, Ruthardt N, Petrášek J, Stierhof K-V, Morris DA, Emans N, Jürgens G, Geldner N, Friml J (2005) Auxin inhibits endocytosis and promotes its own efflux from cells. Nature 435:1251
Petrášek J, Mravec J, Bouchard R, Blakeslee JJ, Abas M, Seifertová D et al (2006) PIN proteins perform a rate-limiting function in cellular auxin efflux. Science 312:914–918
Price J, Li T, Kang S, Na J, Jang J (2003) Mechanisms of glucose signaling during germination of Arabidopsis. Plant Physiol 132:1424–1438
Schrader J, Baba K, May ST, Palme K, Bennett M, Bhalerao RP, Sandberg G (2003) Polar auxin transport in the wood-forming tissues of hybrid aspen is under simultaneous control of developmental and environmental signals. PNAS 100:10096–10101
Serrani JC, Carrera E, Ruiz-Rivero O, Gallego-Giraldo L, Peres LEP, García-Martínez JL (2010) Inhibition of auxin transport from the ovary or from the apical shoot induces parthenocarpic fruit-set in tomato mediated by gibberellins. Plant Physiol 153:851–862
Serrano A, Espinoza C, Armijo G, Inostroza-Blancheteau C, Poblete E, Meyer-Regueiro AA, Parada F, Santibáñez C, Arce-Johnson P (2017) Omics approaches for understanding grapevine berry development: regulatory networks associated with endogenous processes and environmental responses. Front Plant Sci 8:1486
Solfanelli C, Poggi A, Loreti E, Alpi A, Perata P (2006) Sucrose-specific induction of the anthocyanin biosynthetic pathway in Arabidopsis. Plant Physiol 140:637–646
Sun L, Zhang M, Ren QJ, Zhang G, Leng P (2010) Reciprocity between abscisic acid and ethylene at the onset of berry ripening and after harvest. BMC Plant Biol 10:257
Symons GM, Chua YJ, Ross JJ, Quittenden LJ, Davies NW, Reid JB (2012) Hormonal changes during non-climacteric ripening in strawberry. J Exp Bot 63:4741–4750
Taylor JE, Whitelaw CA (2001) Signals in abscission. New Phytol 151:323–339
Thevelein JM, Voordeckers K (2009) Functioning and evolutionary significance of nutrient transceptors. Mol Biol Evol 26:2407–2414
Vega A, Gutiérrez RA, Peña-Neira A, Cramer GR, Arce-Johnson P (2011) Compatible GLRaV-3 viral infections affect berry ripening decreasing sugar accumulation and anthocyanin biosynthesis in Vitis vinifera. Plant Mol Biol 77:261
Vieten A, Vanneste S, Wisniewska J, Benková E, Benjamins R, Beeckman T, Luschnig C, Friml J (2005) Functional redundancy of PIN proteins is accompanied by auxin-dependent cross-regulation of PIN expression. Development 132:4521–4531
Vignault C, Vachaud M, Cakir B, Glissant D, Dédaldéchamp F, Büttner M, Atanassova R, Fleurat-Lessard P, Lemoine R, Delrot S (2005) VvHT1 encodes a monosaccharide transporter expressed in the conducting complex of the grape berry phloem. J Exp Bot 56:1409–1418
Vondras AM, Gouthu S, Schmidt JA, Petersen AR, Deluc LG (2016) The contribution of flowering time and seed content to uneven ripening initiation among fruits within Vitis vinifera L. cv. Pinot Noir Clusters Planta 243:1191–1202
Weiste C, Pedrotti L, Selvanayagam J, Muralidhara P, Fröschel C, Novák O, Ljung K, Hanson J, Dröge-Laser W (2017) The Arabidopsis bZIP11 transcription factor links low-energy signalling to auxin-mediated control of primary root growth. PLoS Genet 13:e1006607
Wheeler S, Loveys B, Ford C, Davies C (2009) The relationship between the expression of abscisic acid biosynthesis genes, accumulation of abscisic acid and the promotion of Vitis vinifera L. berry ripening by abscisic acid. Aust J Grape Wine Res 15:195–204
Wiśniewska J, Xu J, Seifertová D, Brewer PB, Růžička BI, Rouquié D, Benková E, Scheres B, Friml J (2006) Polar PIN localization directs auxin flow in plants. Science 312:883–883
Wuriyanghan H, Zhang B, Cao WH, Ma B, Lei G, Liu YF et al (2009) The ethylene receptor ETR2 delays floral transition and affects starch accumulation in rice. Plant Cell 21:1473–1494
Yang H, Murphy AS (2009) Functional expression and characterization of Arabidopsis ABCB, AUX 1 and PIN auxin transporters in Schizosaccharomyces pombe. Plant J 59:179–191
Yuan TT, Xu HH, Zhang KX, Guo TT, Lu YT (2014) Glucose inhibits root meristem growth via ABA INSENSITIVE 5, which represses PIN1 accumulation and auxin activity in Arabidopsis. Plant Cell Environ 37:1338–1350
Zazimalova E, Murphy AS, Yang H, Hoyerova K, Hosek P (2010) Auxin transporters–why so many? Cold Spring Harb Perspect Biol 2:a001552
Zhang XR, Luo GG, Wang RH, Wang J, Himelrick DG (2003) Growth and developmental responses of seeded and seedless grape berries to shoot girdling. J Am Soc Hortic Sci 128:316–323
Zhu G, Liu Y, Ye N, Liu R, Zhang J (2011) Involvement of the abscisic acid catabolic gene CYP707A2 in the glucose-induced delay in seed germination and post-germination growth of Arabidopsis. Physiol Plant 143:375–384
Ziliotto F, Corso M, Rizzini FM, Rasori A, Botton A, Bonghi C (2012) Grape berry ripening delay induced by a pre-véraison NAA treatment is paralleled by a shift in the expression pattern of auxin-and ethylene-related genes. BMC Plant Biol 12(1):185
Funding
This work was supported by Proyecto Postdoctorado FONDECYT-CONICYT Nº 3150608 and Nº 3150259, ICM-MINECON, Proyecto NC130030, NM BSBSV, and FONDECYT 1150220.
Author information
Authors and Affiliations
Contributions
AS and NK conceived, design, and conducted research. AS, NK FR, and CM-R wrote the manuscript. AS, FR, MM, and PAJ were involved in revising the manuscript critically for important intellectual content. All authors read and approved the final manuscript. We thank Alyssa Grube for assistance in language support.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling Editor: James campanella.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Serrano, A., Kuhn, N., Restovic, F. et al. The Glucose-Related Decrease in Polar Auxin Transport During Ripening and its Possible Role in Grapevine Berry Coloring. J Plant Growth Regul 42, 365–375 (2023). https://doi.org/10.1007/s00344-021-10553-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-021-10553-6