Abstract
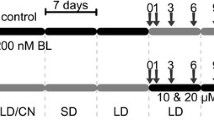
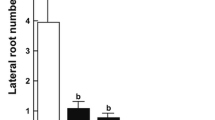
Boron (B) has been considered either as a nutrient or as a toxic non-essential element for plants and considerable debate arose recently regarding its functions and mechanisms of action. To gain further detail on the roles of boron in growth, development, and cell viability, Arabidopsis wild-type and mediator18 mutants, these later showing genetically fixed cell death in the pro-vasculature of roots, were germinated and grown side by side in agar-solidified plates with a standard nutrient solution supplemented with increasing concentrations (0.25–8 mM) of boric acid (BA). In the WT and med18-1 mutants, BA exerted a dose-dependent inhibition of leaf formation and primary root growth, but did not significantly promote root branching in the WT or med18-1 mutants, these later manifesting an enhanced lateral root formation capacity under a wide range of BA concentrations. Although the overall effect of BA in roots was growth repressing, no signs of cell death in meristems of primary roots of WT seedlings could be appreciated, instead, it appears to diminish the cell death manifested in pro-vasculature of med18 mutants, which correlates with reduced expression of the ERF115 transcription factor, which is induced in inner tissues upon damage, and recovery of auxin-inducible gene expression within the root tip. Irrespective of the overall growth-repressing effects in the shoot and root systems, it seems clear that important protective functions are orchestrated by BA, which supports cell viability in a mutant with spontaneous tissue damage.







Similar content being viewed by others
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Code Availability
Not applicable.
References
Aquea F, Federici F, Moscoso C, Vega A, Jullian P, Haseloff J, Arce-Johnson PA (2012) A molecular framework for the inhibition of arabidopsis root growth in response to boron toxicity. Plant Cell Environ 35:719–734
Ayhanci A, Tanriverdi DT, Sahinturk V et al (2020) Protective effects of boron on cyclophosphamide-induced bladder damage and oxidative stress in rats. Biol Trace Elem Res 197:184–191
Bouchareb R, Katz M, Saadallah N et al (2020) Boron improves cardiac contractility and fibrotic remodeling following myocardial infarction injury. Sci Rep 10:17138
Canher B, Heyman J, Savina M et al (2020) Rocks in the auxin stream: wound-induced auxin accumulation and ERF115 expression synergistically drive stem cell regeneration. Proc Natl Acad Sci USA 117:16667–16677
Cohen M, Lepper R (1977) Effect of boron on cell elongation and division in squash roots. Plant Physiol 59:884–887
Feraru E, Feraru MI, Barbez E, Waidmann S, Sun L, Gaidora A, Kleine-Vehn J (2019) PILS6 is a temperature-sensitive regulator of nuclear auxin input and organ growth in Arabidopsis thaliana. Proc Natl Acad Sci USA 116:3893–3898
Furukawa T, Curtis MJ, Tominey CM, Duong YH, Wilcox BW, Aggoune D, Hays JB, Britt AB (2010) A shared DNA-damage-response pathway for induction of stem-cell death by UVB and by gamma irradiation. DNA Repair (amst) 9:940–948
Garnica-Vergara A, Barrera-Ortiz S, Muñoz-Parra E, Raya-González J, Méndez-Bravo A, Macías-Rodríguez L, Ruiz-Herrera L, López-Bucio J (2016) The volatile 6-n-pentyl-2H-pyran-2-one from Trichoderma atroviride regulates Arabidopsis root morphogenesis via auxin signaling and ETHYLENE INSENSITIVE 2 functioning. New Phytol 209:1496–1512
González-Fontes A, Fujiwara T (2020) Advances in plant boron. Int J Mol Sci 21(11):4107
González-Fontes A, Herrera-Rodríguez MB, Martín-Rejano EM, Navarro-Gochicoa MT, Rexach J, Camacho-Cristóbal JJ (2016) Root responses to boron deficiency mediated by ethylene. Front Plant Sci 6:1103
Hanson MR, Köhler RH (2001) GFP imaging: methodology and application to investigate cellular compartmentation in plants. J Exp Bot 52:529–539
Heyman J, Cools T, Vandenbussche F et al (2013) ERF115 controls root quiescent center cell division and stem cell replenishment. Science 342:860–863
Hoermayer L, Montesinos JC, Marhava P, Benková E, Yoshida S, Friml J (2020) Wounding-induced changes in cellular pressure and localized auxin signalling spatially coordinate restorative divisions in roots. Proc Natl Acad Sci USA 117:15322–15331
Hong JH, Savina M, Du J, Devendran A, Kannivadi Ramakanth K, Tian X, Sim WS, Mironova VV, Xu J (2017) A sacrifice-for-survival mechanism protects root stem cell niche from chilling stress. Cell 170:102–113
Hu Z, Cools T, De Veylder L (2016) Mechanisms used by plants to cope with DNA damage. Annu Rev Plant Biol 67:439–462
Huang WN, Liu HK, Zhang HH, Chen Z, Guo YD, Kang YF (2013) Ethylene-induced changes in lignification and cell wall-degrading enzymes in the roots of mungbean (Vignaradiata) sprouts. Plant Physiol Biochem 73:412–419
Kouchi H, Kumazawa K (1975) Anatomical responses of root tips to boron deficiency I. Effects of boron deficiency on elongation of root tips and their morphological characteristics. Soil Sci Plant Nutr 21:21–28
Kutschera U, Niklas KJ (2017) Boron and the evolutionary development of roots. Plant Signal Behav 12(7):20631
Lakehal A, Dob A, Rahneshan Z, Novák O, Escamez S, Alallaq S, Strnad M, Tuominen H, Bellini C (2020) ETHYLENE RESPONSE FACTOR 115 integrates jasmonate and cytokinin signaling machineries to repress adventitious rooting in Arabidopsis. New Phytol 228:1611–1626
Landi M, Remorini D, Pardossi A, Guidi L (2013) Boron excess affects photosynthesis and antioxidant apparatus of greenhouse Cucurbita pepo and Cucumis sativus. J Plant Res 126:775–786
Landi M, Margaritopoulou T, Papadakis IE, Araniti F (2019) Boron toxicity in higher plants: an update. Planta 250:1011–1032
Lewis DH (2019) Boron the essential element for vascular plants that never was. New Phytol 221:1685–1690
Lewis DH (2020) The status of boron as an essential element for vascular plants. I. A response to González-Fontes (2019) ‘Why boron is an essential element for vascular plants.’ New Phytol 226:1231
Li X, Li Y, Mai J, Tao L, Qu M, Liu J et al (2018) Boron alleviates aluminum toxicity by promoting root alkalization in transition zone via polar auxin transport. Plant Physiol 177:1254–1266
Matthes M, Torres-Ruiz RA (2016) Boric acid treatment phenocopies monopteros by affecting PIN1 membrane stability and polar auxin transport in Arabidopsis thaliana embryos. Development 143:4053–4062
Matthes MS, Robil JM, Mc Steen P (2020) From element to development: the power of the essential micronutrient boron to shape morphological processes in plants. J Exp Bot 71:1681–1693
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497
Ortiz-Castro R, López-Bucio J (2019) Review: phytostimulation and root architectural responses to quorum-sensing signals and related molecules from rhizobacteria. Plant Sci 284:135–142
Ortiz-Castro R, Díaz-Pérez C, Martínez-Trujillo M, del Río R, Campos-García J, López-Bucio J (2011) Transkingdom signaling based on bacterial cyclodipeptides with auxin activity in plants. Proc Natl Acad Sci USA 108:7253–7258
Ortiz-Castro R, Pelagio-Flores R, Méndez Bravo A, Ruíz Herrera LF, Campos-García J, López-Bucio J (2014) Pyocyanin, a virulence factor produced by Pseudomonas aeruginosa, alters root development through reactive oxygen species and ethylene signaling in Arabidopsis. Mol Plant-Microbe Interact 27:364–378
Ortiz-Castro R, Campos-García J, López-Bucio J (2020) Pseudomonas putida and Pseudomonas fluorescens influence Arabidopsis root system architecture through an auxin response mediated by bioactive cyclodipeptides. J Plant Growth Regul 39:254–265
Papadakis I, Dimassi K, Bosabalidis A, Therios I, Patakas A, Giannakoula A (2004) Effects of B excess on some physiological and anatomical parameters of ‘Navelina’ orange plants grafted on two rootstocks. Environ Exp Bot 51:247–257
Park M, Li Q, Shcheynikov N, Muallem S, Zeng WZ (2005) Borate transport and cell growth and proliferation: not only in plants. Cell Cycle 4:24–26
Pereira GL, Siqueira JA, Batista-Silva W, Cardoso FB, Nunes-Nesi A, Araújo WL (2021) Boron: more than an essential element for land plants? Front Plant Sci 11:610307
Poza-Viejo L, Abreu I, González-García MP, Allauca P, Bonilla I, Bolaños L et al (2018) Boron deficiency inhibits root growth by controlling meristem activity under cytokinin regulation. Plant Sci 270:176–189
Raya-Gonzalez J, Oropeza-Aburto A, López-Bucio JS, Guevara-Garcia AA, de Veylder L, López-Bucio J, Herrera-Estrella L (2018) MEDIATOR18 influences Arabidopsis root architecture, represses auxin signaling and is a critical factor for cell viability in root meristems. Plant J 96:895–909
Raya-Gonzalez J, Ortiz-Castro R, López-Bucio J (2019) Determinate root development in the halted primary root1 mutant of Arabidopsis correlates with death of root initial cells and an enhanced auxin response. Protoplasma 256:1657–1666
Riaz M, Yana L, Wua X, Hussainb S, Aziza O, Imrana M et al (2018) Boron reduces aluminum-induced growth inhibition, oxidative damage and alterations in the cell wall components in the roots of trifoliate orange. Ecotoxicol Environ Saf 153:107–115
Routray I, Ali S (2019) Boron inhibits apoptosis in hyper-apoptosis condition: acts by stabilizing the mitochondrial membrane and inhibiting matrix remodeling. Biochim Biophys Acta Gen Subj 1863:144–152
Ruiz-Aguilar B, Raya-Gonzalez J, López-Bucio JS, Reyes de la Cruz H, Herrera-Estrella L, Ruiz-Herrera LF, Martinez-Trujillo M, López-Bucio J (2020) Mutation of MEDIATOR 18 and chromate trigger twinning of the primary root meristem in Arabidopsis. Plant Cell Environ 43:1989–1999
Sakamoto T, Tsujimoto-Inui Y, Sotta N, Hirakawa T, Matsunaga TM, Fukao Y et al (2018) Proteasomal degradation of BRAHMA promotes boron tolerance in Arabidopsis. Nat Commun 9:5285
Sarkar AK, Luijten M, Miyashima S, Lenhard M, Hashimoto T, Nakajima K, Scheres B, Heidstra R, Laux T (2007) Conserved factors regulate signalling in Arabidopsis thaliana shoot and root stem cell organizers. Nature 446:811–814
Sjogren CA, Bolaris SC, Larsen PB (2015) Aluminum-dependent terminal differentiation of the Arabidopsis root tip is mediated through an ATR, ALT2, and SOG1-regulated transcriptional response. Plant Cell 27:2501–2515
Tepedelen BE, Soya E, Korkmaz M (2016) Boric acid reduces the formation of DNA double strand breaks and accelerates wound healing process. Biol Trace Elem Res 174:309–318
Wei P, Demulder M, David P, Eekhout T, Yoshiyama KO, Nguyen L, Vercauteren I, Eeckhout D, Galle M, De Jaeger G et al (2021) Arabidopsis casein kinase 2 triggers stem cell exhaustion under Al toxicity and phosphate deficiency through activation of the DNA damage response pathway. Plant Cell. https://doi.org/10.1093/plcell/koab018
Wimmer MA, Abreu I, Bell RW, Bienert MD, Brown PH, Dell B, Fujiwara T, Goldbach HE, Lehto T, Mock HP et al (2020) Boron: an essential element for vascular plants. A response to Lewis (2020) ‘Boron: the essential element for vascular plants that never was.’ New Phytol 226:1232–1237
Xiong Y, Jin E, Yin Q et al (2021) Boron attenuates heat stress-induced apoptosis by inhibiting endoplasmic reticulum stress in mouse granulosa cells. Biol Trace Elem Res 199:611–621
Zhang Q, Li Y, Fu ZY, Liu XB, Yuan K, Fang Y, Liu Y, Li G, Zhang XS, Chong K, Ge L (2018) Intact Arabidopsis RPB1 functions in stem cell niches maintenance and cell cycling control. Plant J 95:150–167
Zhou W, Lozano-Torres JL, Blilou I, Zhang X, Zhai Q, Smant G, Li C, Scheres B (2019) A jasmonate signaling network activates root stem cells and promotes regeneration. Cell 177:942–956
Acknowledgements
We are thankful to Dr. Javier Raya-González for providing us with Arabidopsis mutant seeds.
Funding
This work was financially supported by grants from SEP-CONACYT A1-S-34768 and the Consejo de la Investigación Científica UMSNH (CIC 2.26).
Author information
Authors and Affiliations
Contributions
CETQ and JLB conceived and designed the experiments; CETQ, PIHV, and LFRH performed experiments; CETQ, LFRH, PIHV, and JLB analyzed the data; JLB contributed reagents/materials/analysis tools and wrote the manuscript. All authors read and approved the final draft.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest or competing interests to declare.
Additional information
Handling Editor: Francesca Resentini.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tapia-Quezada, C.E., Ruiz-Herrera, L.F., Huerta-Venegas, P.I. et al. Mild High Concentrations of Boric Acid Repress Leaf Formation and Primary Root Growth in Arabidopsis Seedlings While Showing Anti-apoptotic Effects in a Mutant with Compromised Cell Viability. J Plant Growth Regul 41, 3410–3420 (2022). https://doi.org/10.1007/s00344-021-10523-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-021-10523-y