Abstract
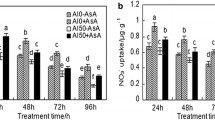
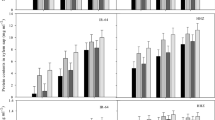
In this study, 5 mM calcium acetate and calcium chloride were individually sprayed twice on rice leaves to study their function in antioxidant response under ozone stress (125 ± 25 ppb). Results showed that elevated O3 during rice cultivation impaired photosystem II (PSII) and carbon dioxide assimilation, as indicated by a decrease in effective quantum yield of PSII and electron transport rate and an increase in intracellular carbon dioxide. This occurrence led to growth inhibition resulting in rice yield and grain size (width and thickness) reduction, accompanied with the increase of malondialdehyde (MDA) content in plant cells under O3 stress. Rice plant pretreated with calcium acetate could endure long-term O3 exposure through the enhancement of NAD kinase activity and NADPH content, leading to an increase in ascorbate peroxidase (APX) and glutathione reductase (GR) activities and higher levels of reduced ascorbic acid (ASA) and reduced glutathione (GSH) under long-term O3 exposure. Consequently, the application of calcium acetate led to increase PSII efficiency and reduce cell damages (MDA content), resulting in an increase in rice yield and grain size under O3 stress. Under long-term O3 exposure, pretreatment of rice with calcium chloride resulted in a decrease in APX activity and an increase in MDA level, resulting in a rice yield similar to that of the non-pretreatment. It suggested that calcium acetate pretreatment of rice was more effective than pretreatment with calcium chloride in terms of reducing stress and maintaining antioxidant enzyme activities, and redox compounds in cells under long-term O3 exposure.






Similar content being viewed by others
Data Availability
All data generated or analyzed during this study are included in this article and can be available from the corresponding author on request.
References
Ahmad P, AbdAllah EF, Alyemeni MN, Wijaya L, Alam P, Bhardwaj R, Siddique KH (2018) Exogenous application of calcium to 24-epibrassinosteroid pre-treated tomato seedlings mitigates NaCl toxicity by modifying ascorbate–glutathione cycle and secondary metabolites. Sci Rep 8(1):1–15
Ainsworth EA (2008) Rice production in a changing climate: a meta-analysis of responses to elevated carbon dioxide and elevated ozone concentration. Glob Change Biol 14(7):1642–1650
Akhtar N, Yamaguchi M, Inada H, Hoshino D, Kondo T, Fukami M, Izuta T (2010) Effects of ozone on growth, yield and leaf gas exchange rates of four Bangladeshi cultivars of rice (Oryza sativa L.). Environ Pollut 158(9):2970–2976
Allan E, Trewavas A (1985) Quantitative changes in calmodulin and NAD kinase during early cell development in the root apex of Pisum sativum L. Planta 165(4):493–501
Alshaal T, El-Ramady H (2017) Foliar application: from plant nutrition to biofortification. EBSS 1(2017):1–83
Anderson JM, Cormier MJ (1978) Calcium-dependent regulator of NAD kinase in higher plants. Biochem Biochem Bioph Res Comun 84(3):595–602
Ashrafuzzaman M, Lubna FA, Holtkamp F, Manning WJ, Kraska T, Frei M (2017) Diagnosing ozone stress and differential tolerance in rice (Oryza sativa L.) with ethylenediurea (EDU). Environ Pollut 230:339–350
Berrin JG, Pierrugues O, Brutesco C, Alonso B, Montillet JL, Roby D, Kazmaier M (2005) Stress induces the expression of AtNADK-1, a gene encoding a NAD(H) kinase in Arabidopsis thaliana. Mol Genet Genom 273(1):10–19
Bilan DS, Shokhina AG, Lukyanov SA, Belousov VV (2015) Main cellular redox couples. Russ J Bioorg Chem 41(4):341–356
Blokhina O, Virolainen E, Fagerstedt KV (2003) Antioxidants, oxidative damage and oxygen deprivation stress: a review. Ann Bot 91(2):179–194
Borchert R (1986) Calcium acetate induces calcium uptake and formation of calcium oxalate crystals in isolated leaflets of Gleditsia triacanthos L. Planta 168:571–578
Brauer M, Freedman G, Frostad J, Van Donkelaar A, Martin RV, Dentener F, Balakrishnan K (2016) Ambient air pollution exposure estimation for the global burden of disease 2013. Environ Sci Technol 50(1):79–88
Chai MF, Chen QJ, An R, Chen YM, Chen J, Wang XC (2005) NADK2, an Arabidopsis chloroplastic NAD kinase, plays a vital role in both chlorophyll synthesis and chloroplast protection. Plant Mol Biol 59(4):553–564
Chen CP, Frei M, Wissuwa M (2011) The OzT8 locus in rice protects leaf carbon assimilation rate and photosynthetic capacity under ozone stress. Plant Cell Environ 34(7):1141–1149
Chen Q, Wang B, Ding H, Zhang J, Li S (2019) The role of NADP-malic enzyme in plants under stress. Plant Sci 281:206–212
Chen Z, Gallie DR (2005) Increasing tolerance to ozone by elevating foliar ascorbic acid confers greater protection against ozone than increasing avoidance. Plant Physiol 138(3):1673–1689
Cho K, Shibato J, Agrawal GK, Jung YH, Kubo A, Jwa NS, Kimura S (2008) Integrated transcriptomics, proteomics, and metabolomics analyses to survey ozone responses in the leaves of rice seedling. J Proteome Res 7(7):2980–2998
Choquette NE, Ainsworth EA, Bezodis W, Cavanagh AP (2020) Ozone tolerant maize hybrids maintain Rubisco content and activity during long-term exposure in the field. Plant Cell Environ 43(12):3033–3047
Corpas FJ, Barroso JB (2014) NADPH-generating dehydrogenases: their role in the mechanism of protection against nitro-oxidative stress induced by adverse environmental conditions. Front Environ Sci 2:55
Delumeau O, Paven MCML, Montrichard F, Laval-Martin DL (2000) Effects of short-term NaCl stress on calmodulin transcript levels and calmodulin-dependent NAD kinase activity in two species of tomato. Plant Cell Environ 23(3):329–336
Dghim AA, Dumont J, Hasenfratz-Sauder MP, Dizengremel P, Le Thiec D, Jolivet Y (2013) Capacity for NADPH regeneration in the leaves of two poplar genotypes differing in ozone sensitivity. Physiol Plant 148(1):36–50
Dongsansuk A, Lontom W, Wannapat S, Theerakulpisut P (2013) The performance of PSII efficiency and growth response to salt stress in three rice varieties differing in salt tolerance. Mol Stress Physiol Plants 44:639–647
Dvorak P, Krasylenko Y, Zeiner A, Samaj J, Takac T (2020) Signaling toward ROS-scavenging enzymes in plants. Front Plant Sci 11:2178
Elavarthi S, Martin B (2010) Spectrophotometric assays for antioxidant enzymes in plants. Plant stress tolerance. Humana Press, Totowa, pp 273–280
Elizabeth A (2017) Understanding and improving global crop response to ozone pollution. Plant J 90(5):886–897
Ellman GE (1959) Tissue sulphydryl groups. Arch Biochem Biophys 82:70–77
Emberson LD, Pleijel H, Ainsworth EA, Van den Berg M, Ren W, Osborne S, Van Dingenen R (2018) Ozone effects on crops and consideration in crop models. Eur J Agron 100:19–34
Farquhar GD, von Caemmerer SV, Berry JA (1980) A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 149(1):78–90
Feng Y, Komatsu S, Furukawa T, Koshiba T, Kohno Y (2008a) Proteome analysis of proteins responsive to ambient and elevated ozone in rice seedlings. Agr Ecosyst Environ 125(1–4):255–265
Feng Z, Kobayashi K, Ainsworth EA (2008b) Impact of elevated ozone concentration on growth, physiology, and yield of wheat (Triticum aestivum L.): a meta-analysis. Glob Change Biol 4(11):2696–2708
Foyer CH, Noctor G (2011) Ascorbate and glutathione: the heart of the redox hub. Plant Physiol 155(1):2–18
Foyer CH, Souriau N, Perret S, Lelandais M, Kunert KJ, Pruvost C, Jouanin L (1995) Overexpression of glutathione reductase but not glutathione synthetase leads to increases in antioxidant capacity and resistance to photoinhibition in poplar trees. Plant Physiol 109(3):1047–1057
Frei M (2015) Breeding of ozone resistant rice: relevance, approaches and challenges. Environ Pollut 197:144–155
Frei M, Tanaka JP, Chen CP, Wissuwa M (2010) Mechanisms of ozone tolerance in rice: characterization of two QTLs affecting leaf bronzing by gene expression profiling and biochemical analyses. J Exp Bot 61(5):1405–1417
Frei M, Kohno Y, Tietze S, Jekle M, Hussein MA, Becker T, Becker K (2012) The response of rice grain quality to ozone exposure during growth depends on ozone level and genotype. Environ Pollut 163:199–206
Gao H, Xu X (2012) The cyanobacterial NAD kinase gene sll1415 is required for photoheterotrophic growth and cellular redox homeostasis in Synechocystis sp. strain PCC 6803. J Bacteriol 194(2):218–224
Gerdes SY, Scholle MD, D’Souza M, Bernal A, Baev MV, Farrell M, Osterman AL (2002) From genetic footprinting to antimicrobial drug targets: examples in cofactor biosynthetic pathways. J Bacteriol 184(16):4555–4572
Grant GA (2018) Elucidation of a self-sustaining cycle in Escherichia coli l-serine biosynthesis that results in the conservation of the coenzyme, NAD+. Biochemistry 57(11):1798–1806
Grose JH, Joss L, Velick SF, Roth JR (2006) Evidence that feedback inhibition of NAD kinase controls responses to oxidative stress. Proc Natl Acad Sci 103(20):7601–7606
Halliwell B, Foyer CH (1978) Properties and physiological function of a glutathione reductase purified from spinach leaves by affinity chromatography. Planta 139(1):9–17
Harshavardhan VT, Wu TM, Hong CY (2017) Glutathione reductase and abiotic stress tolerance in plants. Glutathione in plant growth, development, and stress tolerance. Springer, Cham, pp 265–286
Hossain MA, Mostofa MG, Diaz-Vivancos P, Burritt DJ, Fujita M, Tran LSP (2017) Glutathione in plant growth, development, and stress tolerance. Springer, Cham
IPCC (2007). In: Solomon S, Qin D, Manning M, Chen Z, Marquis M, Averyt KB, Tignor M, Miller HL (eds) Climate change: the physical science basis. Contribution of working group I to the fourth assessment report of the intergovernmental panel on climate change. Cambridge University Press, Cambridge, p 996
Issam N, Kawther M, Haythem M, Moez J (2012) Effects of CaCl2 pretreatment on antioxidant enzyme and leaf lipid content of faba bean (Vicia faba L.) seedlings under cadmium stress. Plant Growth Regul 68(1):37–47
Kangasjärvi J, Jaspers P, Kollist H (2005) Signaling and cell death in ozone-exposed plants. Plant Cell Environ 28(8):1021–1036
Kittipornkul P, Treesubsuntorn C, Thiravetyan P (2020) Effect of exogenous catechin and salicylic acid on rice productivity under ozone stress: the role of chlorophyll contents, lipid peroxidation, and antioxidant enzymes. Environ Sci Pollut Res 27(20):25774–25784
Kollist H, Moldau H, Oksanen E, Vapaavuori E (2001) Ascorbate transport from the apoplast to the symplast in intact leaves. Physiol Plantarum 113(3):377–383
Kraemer T, Hunsche M, Noga G (2009) Cuticular calcium penetration is directly related to the area covered by calcium within droplet spread area. Sci Hortic 120(2):201–206
Lakaew K, Akeprathumchai S, Thiravetyan P (2020) Foliar spraying of calcium acetate alleviates yield loss in rice (Oryza sativa L.) by induced anti-oxidative defence system under ozone and heat stresses. Ann Appl Biol 178:414–426
Li BB, Wang X, Tai L, Ma TT, Shalmani A, Liu WT, Chen KM (2018) NAD kinases: metabolic targets controlling redox co-enzymes and reducing power partitioning in plant stress and development. Front Plant Sci 9:379
Li S, Courbet G, Ourry A, Ainsworth EA (2019) Elevated ozone concentration reduces photosynthetic carbon gain but does not alter leaf structural traits, nutrient composition or biomass in switchgrass. Plants 8(4):85
Ma Y, Ren X, Liang C (2021) Exogenous Ca2+ enhances antioxidant defense in rice to simulated acid rain by regulating ascorbate peroxidase and glutathione reductase. Planta 254(2):1–16
Mailloux RJ, Lemire J, Appanna VD (2011) Metabolic networks to combat oxidative stress in Pseudomonas fluorescens. Anton Leeuwenhoek 99(3):433–442
Matsumoto H, Tanigawa M, Tomoyuki Y (1983) Calmodulin-like activity associated with chromatin from pea buds. Plant Cell Physiol 24(4):593–602
McGuinness ET, Butler JR (1985) NAD+ kinase—a review. Int J Biochem 17(1):1–11
Morales M, Munné-Bosch S (2019) Malondialdehyde: facts and artifacts. Plant Physiol 180(3):1246–1250
Mori S, Kawai S, Shi F, Mikami B, Murata K (2005) Molecular conversion of NAD kinase to NADH kinase through single amino acid residue substitution. J Biol Chem 280(25):24104–24112
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22(5):867–880
Noctor G (2006) Metabolic signalling in defence and stress: the central roles of soluble redox couples. Plant Cell Environ 29(3):409–425
Noctor G, Queval G, Gakière B (2006) NAD (P) synthesis and pyridine nucleotide cycling in plants and their potential importance in stress conditions. J Exp Bot 57(8):1603–1620
Noctor G, Queval G, Mhamdi A, Chaouch S, Foyer CH (2011) Glutathione. Arabidopsis Book 9:e0142
Paciolla C, Paradiso A, De Pinto MC (2016) Cellular redox homeostasis as central modulator in plant stress response. Redox state as a central regulator of plant-cell stress responses. Springer, Cham, pp 1–23
Rahman A, Mostofa MG, Alam M, Nahar K, Hasanuzzaman M, Fujita M (2015) Calcium mitigates arsenic toxicity in rice seedlings by reducing arsenic uptake and modulating the antioxidant defense and glyoxalase systems and stress markers. BioMed Res Int 2015:340812
Rahman A, Mostofa MG, Nahar K, Hasanuzzaman M, Fujita M (2016) Exogenous calcium alleviates cadmium-induced oxidative stress in rice (Oryza sativa L.) seedlings by regulating the antioxidant defense and glyoxalase systems. Braz J Bot 39(2):393–407
Rao MV, Davis KR (2001) The physiology of ozone induced cell death. Planta 213:682–690
Romanowska-Duda Z, Grzesik M, Janas R (2019) Maximal efficiency of PSII as a marker of sorghum development fertilized with waste from a biomass biodigestion to methane. Front Plant Sci 9:1920
Ruiz JM, Sanchez E, Garcıa PC, Lopez-Lefebre LR, Rivero RM, Romero L (2002) Proline metabolism and NAD kinase activity in greenbean plants subjected to cold-shock. Phytochemistry 59(5):473–478
Sagi M, Fluhr R (2006) Production of reactive oxygen species by plant NADPH oxidases. Plant Physiol 141(2):336–340
Sawada H, Komatsu S, Nanjo Y, Khan NA, Kohno Y (2012) Proteomic analysis of rice response involved in reduction of grain yield under elevated ozone stress. Environ Exp Bot 77:108–116
Sawada H, Tsukahara K, Kohno Y, Suzuki K, Nagasawa N, Tamaoki M (2016) Elevated ozone deteriorates grain quality of Japonica Rice cv. Koshihikari, even if it does not cause yield reduction. Rice 9(1):1–10
Schlegel TK, Schönherr J (2001) Selective permeability of cuticles over stomata and trichomes to calcium chloride. Int Symp Foliar Nutr Perenn Fruit Plants 594:91–96
Shao Z, Zhang Y, Mu H, Wang Y, Wang Y, Yang L (2020) Ozone-induced reduction in rice yield is closely related to the response of spikelet density under ozone stress. Sci Total Environ 712:136560
Simon P, Dieter P, Bonzon M, Greppin H, Marmé D (1982) Calmodulin-dependent and independent NAD kinase activities from cytoplasmic and chloroplastic fractions of spinach (Spinacia oleracea L.). Plant Cell Rep 1(3):119–122
Singh R, Mailloux RJ, Puiseux-Dao S, Appanna VD (2007) Oxidative stress evokes a metabolic adaptation that favors increased NADPH synthesis and decreased NADH production in Pseudomonas fluorescens. J Bacteriol 189(18):6665–6675
Singh A, Banerjee A, Roychoudhury A (2019) Seed priming with calcium compounds abrogate fluoride-induced oxidative stress by upregulating defence pathways in an indica rice variety. Protoplasma 257(3):767–782
Slaski JJ (1989) Effect of aluminium on calmodulin-dependent and calmodulin-independent NAD kinase activity in wheat (Triticum aestivum L.) root tips. J Plant Physiol 133(6):696–701
Smith IK, Vierheller TL, Thorne CA (1988) Assay of glutathione reductase in crude tissue homogenates using 5, 5′-dithiobis (2-nitrobenzoic acid). Anal Biochem 175(2):408–413
Soares C, Carvalho ME, Azevedo RA, Fidalgo F (2019) Plants facing oxidative challenges—a little help from the antioxidant networks. Environ Exp Bot 161:4–25
Srivastava RK, Pandey P, Rajpoot R, Rani A, Gautam A, Dubey R (2015) Exogenous application of calcium and silica alleviates cadmium toxicity by suppressing oxidative damage in rice seedlings. Protoplasma 252(4):959–975
Tagnon MD, Simeon KO (2017) Aldehyde dehydrogenases may modulate signaling by lipid peroxidation-derived bioactive aldehydes. Plant Signal Behav 12(11):e1387707
Tahjib-Ul-Arif M, Roy PR, Sohag AAM, Afrin S, Rady MM, Hossain MA (2018) Exogenous calcium supplementation improves salinity tolerance in BRRI dhan28; a salt-susceptible high-yielding Oryza sativa cultivar. J Crop Sci Biotechnol 21(4):383–394
Tai L, Li BB, Nie XM, Zhang PP, Hu CH, Zhang L, Chen KM (2019) Calmodulin is the fundamental regulator of NADK-mediated NAD signaling in plants. Front Plant Sci 10:681
Takahashi H, Takahara K, Hashida SN, Hirabayashi T, Fujimori T, Kawai-Yamada M, Uchimiya H (2009) Pleiotropic modulation of carbon and nitrogen metabolism in Arabidopsis plants overexpressing the NAD kinase2 gene. Plant Physiol 151(1):100–113
Takahara K, Kasajima I, Takahashi H, Hashida SN, Itami T, Onodera H, Uchimiya H (2010) Metabolome and photochemical analysis of rice plants overexpressing Arabidopsis NAD kinase gene. Plant Physiol 152(4):1863–1873
Tatsumi K, Abiko T, Kinose Y, Inagaki S, Izuta T (2019) Effects of ozone on the growth and yield of rice (Oryza sativa L.) under different nitrogen fertilization regimes. Environ Sci Pollut Res 26(31):32103–32113
The Royal Society (2008) Ground-level ozone in the 21st century: future trends, impacts and policy implications. The Royal Society, London
Thwe AA, Vercambre G, Gautier H, Gay F, Phattaralerphong J, Kasemsap P (2014) Response of photosynthesis and chlorophyll fluorescence to acute ozone stress in tomato (Solanum lycopersicum Mill.). Photosynthetica 52(1):105–116
Wang Y, Yang L, Höller M, Zaisheng S, Pariasca-Tanaka J, Wissuwa M, Frei M (2014) Pyramiding of ozone tolerance QTLs OzT8 and OzT9 confers improved tolerance to season-long ozone exposure in rice. Environ Exp Bot 104:26–33
Wang X, Li BB, Ma TT, Sun LY, Tai L, Hu CH, Chen KM (2020) The NAD kinase OsNADK1 affects the intracellular redox balance and enhances the tolerance of rice to drought. BMC Plant Biol 20(1):1–19
White PJ, Broadley MR (2003) Calcium in plants. Ann Bot 92(4):487–511
Wigge B, Krömer S, Gardeström P (1993) The redox levels and subcellular distribution of pyridine nucleotides in illuminated barley leaf protoplasts studied by rapid fractionation. Physiol Plant 88(1):10–18
Wilkinson S, Mills G, Illidge R, Davies WJ (2012) How is ozone pollution reducing our food supply? J Exp Bot 63(2):527–536
Xu C, Li X, Zhang L (2013) The effect of calcium chloride on growth, photosynthesis, and antioxidant responses of Zoysia japonica under drought conditions. PLoS ONE 8(7):e68214
Yamauchi Y, Sugimoto Y (2010) Effect of protein modification by malondialdehyde on the interaction between the oxygen-evolving complex 33 kDa protein and photosystem II core proteins. Planta 231(5):1077–1088
Yang H, Jie Y (2005) Uptake and transport of calcium in plants. Physiol Mol Biol Plants 31(3):227
Yousuf PY, Hakeem KUR, Chandna R, Ahmad P (2012) Role of glutathione reductase in plant abiotic stress. Abiotic stress responses in plants. Springer, New York, pp 149–158
Yuan X, Calatayud V, Jiang L, Manning WJ, Hayes F, Tian Y, Feng Z (2015) Assessing the effects of ambient ozone in China on snap bean genotypes by using ethylenediurea (EDU). Environ Pollut 205:199–208
Zeng H, Xu L, Singh A, Wang H, Du L, Poovaiah BW (2015) Involvement of calmodulin and calmodulin-like proteins in plant responses to abiotic stresses. Front Plant Sci 6:600
Zheng YH, Li X, Li YG, Miao BH, Xu H, Simmons M, Yang XH (2012) Contrasting responses of salinity-stressed salt-tolerant and intolerant winter wheat (Triticum aestivum L.) cultivars to ozone pollution. Plant Physiol Biochem 52:169–178
Zheng F, Wang X, Zhang W, Hou P, Lu F, Du K, Sun Z (2013) Effects of elevated O3 exposure on nutrient elements and quality of winter wheat and rice grain in Yangtze River Delta, China. Environ Pollut 179:19–26
Acknowledgements
The authors would like to express their gratitude to King Mongkut's University of Technology Thonburi for financially supporting Mr. Kittisak Lakaew through the Petchra Pra Jom Klao PhD scholarship, Grant No. 16/2559.
Funding
This study was funded by Petchra Pra Jom Klao PhD scholarship, King Mongkut's University of Technology Thonburi, Thailand (Grant No. 16/2559).
Author information
Authors and Affiliations
Contributions
PT and SA provided the idea, advised and discussed the entire work. KL designed, conducted the experiment and drafted the manuscript. All the authors analyzed and interpreted the data and improved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Consent of Participate
Not applicable.
Consent to Publish
Not applicable.
Ethical Approval
Not applicable.
Additional information
Handling Editor: Mikihisa Umehara.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lakaew, K., Akeprathumchai, S. & Thiravetyan, P. Effect of Calcium Acetate and Calcium Chloride on Grain Morphology and Antioxidant Regulation in rice Under Ozone Stress. J Plant Growth Regul 41, 3138–3152 (2022). https://doi.org/10.1007/s00344-021-10501-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-021-10501-4