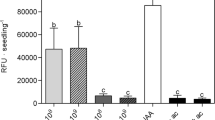
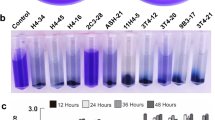
Abstract
Plant growth-promoting rhizobacteria modulate root development through different mechanisms. This work was conducted to evaluate the effects of root colonization by Pseudomonas putida and Pseudomonas fluorescens in biomass production, lateral root formation, and activation of auxin signaling in Arabidopsis thaliana. Selected strains of P. putida and P. fluorescens were tested for modification of DR5::uidA, BA3::uidA and HS::AXR3NT-GUS auxin-related gene expression, and to promote root hair and lateral root formation in WT and tir1-1, tir1-1afb2-1afb3-1, arf7-1, arf19-1, arf7-1arf19-1, and rhd6 mutants. Production of cyclodipeptides with possible roles in auxin signaling was also determined in P. putida and P. fluorescens culture supernatants by gas chromatography–mass spectrometry. P. putida and P. fluorescens stimulated lateral root and root hair formation and increased plant biomass, which correlated with an induction of the auxin response. Genetic analyses suggested that growth promotion involves auxin signaling as tir1-1, tir1-1afb2-1afb3-1, arf7-1, arf19-1, and arf7-1arf19-1 mutants showed decreased lateral root response to inoculation and because P. putida and P. fluorescens restored root hair development in the rhd6 mutant. It was also found that these bacteria produce the cyclodipeptides cyclo(L-Pro-L-Val), cyclo(L-Pro-L-Phe), and cyclo(L-Pro-L-Tyr), which modulates auxin-responsive gene expression in roots. Our results suggest a role of cyclodipeptides for bacterial phytostimulation.






Similar content being viewed by others
Abbreviations
- AHL:
-
N-Acyl-l-homoserine lactones
- ARFs:
-
Auxin response transcription factors
- CDPs:
-
Cyclodipeptides
- DAPG:
-
2, 4-Diacetylphloroglucinol
- IAA:
-
Indole-3-acetic acid
- LRD:
-
Lateral root density
- LRN:
-
Lateral root number
- LRP:
-
Lateral root primordia
- PGPR:
-
Plant growth-promoting rhizobacteria
- QS:
-
Quorum-sensing
- TIR1/AFB:
-
Transport inhibitor response/auxin receptor F-box proteins
References
Aguilar JA, Zavala AN, Díaz-Pérez C, Cervántes C, Díaz-Pérez AL, Campos-García J (2006) The atu and liu clusters are involved in the catabolic pathways for acyclic monoterpenes and leucine in Pseudomonas aeruginosa. Appl Environ Microbiol 72:2070–2079
Badri DV, Weir TL, Van der Lelie D, Vivanco JM (2009) Rhizosphere chemical dialogues: plant-microbe interactions. Curr Opin Biotech 20:642–650
Barazani O, Friedman J (1999) Is IAA the major root growth factor secreted from plant-growth-mediating bacteria? J Chem Ecol 25:2397–2406
Barlier I, Kowalczyk M, Marchant A, Ljung K, Bhalerao R, Bennett M, Sandberg G, Bellini C (2000) The SUR2 gene of Arabidopsis thaliana encodes the cytochrome P450 CYP83B1, a modulator of auxin homeostasis. Proc Natl Acad Sci USA 97:14819–14824
Benková E, Michniewicz MSM, Teichmann T, Seifertova D, Jürgens G, Friml J (2003) Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 115:591–602
Blakely L, Evans T (1979) Cell dynamics studies on the pericycle of radish seedling roots. Plant Sci Lett 14:79–83
Braud A, Jézéquel K, Bazot S, Lebeau T (2009) Enhanced phytoextraction of an agricultural Cr-, Hg- and Pb-contaminated soil by bioaugmentation with siderophoreproducing bacteria. Chemosphere 74:280–286
Brazelton JN, Pfeufer EE, Sweat TA, Gardener BB, Coenen C (2008) 2,4-Diacetylphloroglucinol alters plant root development. Mol Plant-Microbe Interact 21:1349–1358
Campbell J, Lin Q, Geske GD, Blackwell HE (2009) New and unexpected insights into the modulation of LuxR-type quorum sensing by cyclic dipeptides. ACS Chem Biol 4:1051–1059
Carrillo-Castaneda G, Munoz JJ, Peralta-Videa JR, Gomez E, Gardea-Torresdey JL (2003) Plant growth-promoting bacteria promote copper and iron translocation from root to shoot in alfalfa seedlings. J Plant Nutr 26:1801–1814
Colón-Carmona A, You R, Haimovitch-Gal T, Doermer P (1999) Spatio-temporal analysis of mitotic activity with a labile cyclin-GUS fusion protein. Plant J 20:503–508
Contesto C, Milesi S, Mantelin S, Zancarini A, Desbrosses G, Varoquaux F, Bellini C, Kowalczyk M, Touraine B (2010) The auxin-signaling pathway is required for the lateral root response of Arabidopsis to the rhizobacterium Phyllobacterium brassicacearum. Planta 232:1455–1470
Dey R, Pal KK, Bhat DM (2004) Grwoth promotion and yield enhancement of peanut (Arachis hypogaea L.) by application of plant growth-promoting rhizobacteria. Microbiol Res 159:371–394
Dharmasiri N, Dharmasiri S, Weijers D, Lechner E, Yamada M, Hobbie L, Ehrismann J, Jürgens G, Estelle M (2005) Plant development is regulated by a family of auxin receptor F box proteins. Dev Cell 9:109–119
Dobblelaere SA, Croonenborghs A, Thys A, Vanden Broek A, Vanderleyden J (1999) Phytostimulatory effect of Azospirillum brasilense wild type and mutant strains altered in IAA production on wheat. Plant Soil 212:155–164
Fukaki H, Tameda S, Masuda H, Tasaka M (2002) Lateral root formation is blocked by a gain-of-function mutation in the SOLITARY-ROOT/IAA14 gene of Arabidopsis. Plant J 29:153–168
Gholami A, Shahsavani S, Nezarat S (2009) The effect of plant growth promoting rhizobacteria (PGPR) on germination, seedling growth and yield of maize. Int J Biol Life Sci 1:35–40
Goh T, Kasahara H, Mimura T, Kamiya Y, Fukaki H (2012) Multiple AUX/IAA-ARF modules regulate lateral root formation: the role of Arabidopsis SHY2/IAA3-mediated auxin signalling. Phil Trans R Soc B 367:1461–1468
González O, Ortiz-Castro R, Díaz-Pérez C, Díaz-Pérez AL, Magaña-Dueñas V, López-Bucio J, Campos-García J (2017) Non-ribosomal peptide synthases from Pseudomonas aeruginosa play a role in cyclodipeptide biosynthesis, quorum-sensing regulation, and root development in a plant host. Microb Ecol 73:616–629
Gray WM (2004) Hormonal regulation of plant growth and development. PLoS Biol 2:1270–1273
Gupta A, Rai V, Bagdwal N, Goel R (2005) In situ characterization of mercury resistant growth promoting fluorescent pseudomonads. Microbiol Res 160:385–388
Jefferson RA, Kavanagh TA, Bevan MW (1987) GUS fusion: b-glucuronidase as a sensitive and versatile fusion marker in higher plants. EMBO J 6:3901–3907
Kepinski S, Leyser O (2005) The Arabidopsis F-box protein TIR1 is an auxin receptor. Nature 435:446–451
Kloepper JW, Zablotowivz RM, Tipping EM, Lifshitz R (1990) Plant growth promotion mediated by bacterial rhizosphere colonizers. In: Keister DL, Cregan PB (eds) The rhizosphere and plant growth. Kluwer Academic, Dordrecht, pp 315–326
López-Bucio J, Cruz-Ramirez A, Herrera-Estrella L (2003) The role of nutrient availability in regulating root architecture. Curr Opin Plant Biol 6:280–287
López-Bucio J, Campos-Cuevas JC, Hernández-Calderón E, Velasquez-Becerra C, Farías-Rodríguez R, Macías-Rodriguez LI, Valencia-Cantero E (2007) Bacillus megaterium rhizobacteria promote growth and alter root-system architecture through an auxin- and ethylene-independent signaling mechanism in Arabidopsis thaliana. Mol Plant-Microbe Interact 20:207–217
Malamy JF, Benfey PN (1997) Organization and cell differentiation in lateral roots of Arabidopsis thaliana. Development 124:33–44
Martínez-Morales LJ, Soto-Urzúa L, Baca BE, Sánchez-Ahédo JA (2003) Indole-3 butyric acid (IBA) production in culture medium by wild strain Azospirillum brasilense. FEMS Microbiol Lett 228:167–173
Masucci JD, Schiefelbein JW (1994) The rhd6 mutation of Arabidopsis thaliana alters root hair initiation through an auxin and ethylene associated process. Plant Physiol 106:1335–1346
Nibau C, Gibbs DJ, Coates JC (2008) Branching out in new directions: the control of root architecture by lateral root formation. New Phytol 179:595–614
Okushima Y, Fukaki H, Onoda M, Theologis A, Tasaka M (2007) ARF7 and ARF19 regulate lateral root formation via direct activation of LBD/ASL genes in Arabidopsis. Plant Cell 19:118–130
Oono Y, Chen QG, Overvoorde PJ, Kôhler C, Theologis A (1998) age Mutants of Arabidopsis exhibit altered auxin-regulated gene expression. Plant Cell 10:1649–1662
Ortiz-Castro R, Martínez-Trujillo M, López-Bucio J (2008) N-acyl-L-homoserine lactones: a class of bacterial quorum-sensing signals alter post-embryonic root development in Arabidopsis thaliana. Plant Cell Environ 31:1497–1509
Ortiz-Castro R, Contreras-Cornejo HA, Macías-Rodríguez L, López-Bucio J (2009) The role of microbiol signals in plant growth and development. Plant Signal Behav 4:1–12
Ortiz-Castro R, Díaz-Pérez C, Martínez-Trujilo M, del Río R, Campos-García J, López-Bucio J (2011) Transkingdom signaling based on bacterial cyclodipeptides with auxin activity in plants. Proc Natl Acad Sci USA 108:7253–7258
Ortiz-Castro R, Campos-García J, López-Bucio J (2013) Rapid identification of plant-growth-promoting rhizobacteria using an agar plate cocultivation system with Arabidopsis. In: de Bruijn FJ (ed) Molecular microbiol ecology of the rhizosphere. Wiley, New Jersey, pp 345–353
Plotnikova JM, Rahme LG, Ausubel FM (2000) Pathogenesis of the human opportunistic pathogen Pseudomonas aeruginosa PA14 in Arabidopsis. Plant Physiol 124:1766–1774
Preston GM (2004) Plant perceptions of plant growth promoting Pseudomonas. Phil Trans R Soc Lond B 359:907–918
Rudrappa T, Czymmek KJ, Paré PW, Bais HP (2008) Root-secreted malic acid recruits beneficia soil bacteria. Plant Physiol 148:1547–1556
Sieberer T, Hauser MT, Seifert GJ, Lusching C (2003) PROPORZ1, a putative Arabidopsis transcriptional adaptor protein, mediates auxin and cytokinin signals in the control of cell proliferation. Curr Biol 13:837–842
Spaepen S, Vanderleyden J, Remans R (2007) Indole-3-acetic acid in microbial and microorganism-plant signaling. FEMS Microbiol Rev 31:425–448
Tripathi M, Munot HP, Schouch Y, Meyer JM, Goel R (2005) Isolation and functional characterization of siderophore-producing lead- and cadmium-resistant Pseudomonas putida KNP9. Curr Microbiol 5:233–237
Ulmasov T, Murfett J, Hagen G, Guilfoyle TJ (1997) AUX/IAA proteins repress expression of report genes containing natural and highly active synthetic auxin response elements. Plant Cell 9:1963–1971
Venturi V (2006) Regulation of quorum sensing in Pseudomonas. FEMS Microbiol Rev 30:274–291
Von Rad U, Klein I, Dobrev PI, Kottova J, Zazimalova E, Fekete A, Hartmann A, Schmitt-Kopplin P, Durner J (2008) Response of Arabidopsis thaliana to N-hexanoyl-DL-homoserine lactone, a bacterial quorum sensing molecule produced in the rhizosphere. Planta 229:73–85
Walker TS, Bais HP, Déziel E, Schweizer HP, Rahme LG, Fall R, Vivanco JM (2004) Pseudomonas aeruginosa-plant root interactions. Pathogenicity, biofilm formation, and root exudation. Plant Physiol 134:320–331
Wang R, Estelle M (2014) Diversity and specificity: auxin perception and signaling through the TIR1/AFB pathway. Curr Opin Plant Biol 21:51–58
Yan PS, Song Y, Sakuno E, Nakajima H, Nakagawa H, Yabe K (2004) Cyclo(L-leucyl-L-prolyl) produced by Achromobacter xylosoxidans inhibits aflatoxin production by Aspergillus parasiticus. Appl Environ Microbiol 70:7466–7473
Zamioudis C, Mastranesti P, Dhonukshe P, Blilou I, Pieterse CMJ (2013) Unraveling root development programs initiated by beneficial Pseudomonas spp. Bacteria Plant Physiol 162:304–318
Zhang H, Jennings A, Barlow PW, Forde BG (1999) Dual pathways for regulation of root branching by nitrate. Proc Natl Acad Sci USA 96:6529–6534
Acknowledgements
We thank Drs. Peter Doerner, Christian Luschnig, Athanasios Theologis, Tom Guilfoyle, and Mark A. Estelle for kindly providing seeds of transgenic and mutant lines. This work was supported by grants from the Consejo Nacional de Ciencia y Tecnología (CONACYT, México, Grant No. 80916, 177775, and PDCPN 2015-882), Consejo de la Investigación Científica (UMSNH, México, Grant No. CIC 2.26), and the Marcos Moshinsky Foundation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
344_2019_9979_MOESM1_ESM.eps
Supplementary material 1 Fig. S1. Effect of co-inoculation of P. putida and P. fluorescens on root hair development in Arabidopsis (WT) seedlings. (A–C) Arabidopsis WT (Col-0) seedlings uninoculated or co-cultivated with P. putida or P. fluorescens. (D) Root hair number, (E) Root hair length. Data represent the mean ± SD (n=100). Different letters indicate means statistically different at P<0.05. (Scale bar = 500 µm). (EPS 143303 kb)
344_2019_9979_MOESM2_ESM.eps
Supplementary material 2 Fig. S2. Effect of co-cultivation with P. putida and P. fluorescens on root hair development in WT (Col-0) seedlings and auxin-related mutants. Arabidopsis WT (Col-0), tir1-1, arf7-1arf19-1 and tir1-1afb2-1afb3-1 mutants seedlings were co-cultivated with P. putida or P. fluorescens at a distance of 5 cm from the primary root tip and grown for additional time. Representative photographs of axenically grown Arabidopsis seedlings or seedlings co-cultivated with P. putida or P. fluorescens (Scale bar = 500 µm). (EPS 105728 kb)
344_2019_9979_MOESM3_ESM.eps
Supplementary material 3 Fig. S3. Effect of co-cultivation with P. putida and P. fluorescens on root hair development of rhd6 Arabidopsis mutant. Arabidopsis rhd6 mutant seedlings were co-cultivated with P. putida or P. fluorescens at a distance of 5 cm from the primary root tip and grown for additional time. Representative photographs of axenic Arabidopsis seedlings or seedlings co-cultivated with P. putida or P. fluorescens (Scale bar = 500 µm) (EPS 76917 kb)
344_2019_9979_MOESM4_ESM.eps
Supplementary material 4 Fig. S4. Effects of cell-free extracts produced by P. putida and P. fluorescens on root system architecture of Arabidopsis WT (Col-0) seedlings and auxin-related mutants. Arabidopsis WT (Col-0) and tir1-1, arf7-1, arf19-1, arf7-1arf19-1 and tir1-1afb2-1afb3-1 mutant seedlings were germinated and grown for 6 days on 0.2x MS medium and then transferred into 24-well cell culture plates (15 seedlings per well) containing 2 mL 0.2x MS liquid medium supplied with the indicated concentrations of compounds and incubated for 12 h. Data represent mean ± SD (n=15). Different letters indicate means statistically different at P<0.05. (EPS 166479 kb)
Rights and permissions
About this article
Cite this article
Ortiz-Castro, R., Campos-García, J. & López-Bucio, J. Pseudomonas putida and Pseudomonas fluorescens Influence Arabidopsis Root System Architecture Through an Auxin Response Mediated by Bioactive Cyclodipeptides. J Plant Growth Regul 39, 254–265 (2020). https://doi.org/10.1007/s00344-019-09979-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-019-09979-w