Abstract
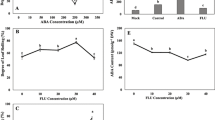
Hydrogen gas (H2) was recently proposed as a novel gaseous signaling molecule. In our previous study, H2-mediated enhancement of plant tolerance to drought stress was preliminarily suggested. However, the detailed mechanisms of the action of H2 have not been fully explored. In this study, we observed that H2 production and hydrogenase activity were significantly induced by abscisic acid (ABA) and drought stress. Alfalfa seedlings pretreated with hydrogen-rich water (HRW) were hypersensitive to exogenous ABA. In response to ABA or water deficit, HRW-pretreated seedlings rapidly accumulated higher amounts of hydrogen peroxide (H2O2), and exhibited more tolerance to drought stress. By contrast, the inhibition or scavenging of H2O2 reduced HRW-induced drought tolerance. Further results showed that the apoplastic pH of leaves was significantly increased by HRW and/or drought stress. Cotreatment with the H+-ATPase inhibitor, however, could prevent the effects of H2 on the alkalinization of the apoplastic sap and stomatal sensitivity to exogenous ABA or water deficit. These responses were interpreted as an effect of H2 on sap pH and closure of stomata in alfalfa via an ABA-based mechanism. Overall, these results suggested a novel regulating mechanism of H2 in plant drought response.




Similar content being viewed by others
References
An Z, Jing W, Liu Y, Zhang W (2008) Hydrogen peroxide generated by copper amine oxidase is involved in abscisic acid-induced stomatal closure in Vicia faba. J Exp Bot 9:815–825
Bright J, Desikan R, Hancock JT, Weir IS, Neill SJ (2006) ABA-induced NO generation and stomatal closure in Arabidopsis are dependent on H2O2 synthesis. Plant J 45:113–122
Cho D, Villiers F, Kroniewicz L, Lee S, Seo YJ, Hirschi KD, Leonhardt N, Kwak JM (2012) Vacuolar CAX1 and CAX3 influence auxin transport in guard cells via regulation of apoplastic pH. Plant Physiol 160:1293–1302
Cui W, Gao C, Fang P, Lin G, Shen W (2013) Alleviation of cadmium toxicity in Medicago sativa by hydrogen-rich water. J Hazard Mater 260:715–724
Cui W, Fang P, Zhu K, Mao Y, Gao C, Xie Y, Wang J, Shen W (2014) Hydrogen-rich water confers plant tolerance to mercury toxicity in alfalfa seedlings. Ecotoxicol Environ Saf 105:103–111
Davies WJ, Zhang J (1991) Root signals and the regulation of growth and development of plants in drying soil. Annu Rev Plant Physiol Plant Mol Biol 42:55–76
Desikan R, Cheung MK, Bright J, Henson D, Hancock JT, Neill SJ (2004) ABA, hydrogen peroxide and nitric oxide signalling in stomatal guard cells. J Exp Bot 55:205–212
Frey M (2002) Hydrogenases: hydrogen-activating enzymes. ChemBioChem 3:153–160
Fromard L, Babin V, Fleurat-Lessard P, Fromont JC, Serrano R, Bonnemain JL (1995) Control of vascular sap pH by the vessel-associated cells in woody species. Plant Physiol 108:913–918
García-Mata C, Lamattina L (2010) Hydrogen sulphide, a novel gasotransmitter involved in guard cell signaling. New Phytol 188:977–984
Hartung W, Sauter A, Hose E (2002) Abscisic acid in the xylem: where does it come from, where does it go to? J Exp Bot 53:27–32
Hong Y, Chen S, Zhang JM (2010) Hydrogen as a selective antioxidant: a review of clinical and experimental studies. J Int Med Res 38:1893–1903
Jia W, Davies WJ (2007) Modification of leaf apoplastic pH in relation to stomatal sensitivity to root-sourced abscisic acid signals. Plant Physiol 143:68–77
Jin Q, Zhu K, Cui W, Xie Y, Han B, Shen W (2013) Hydrogen gas acts as a novel bioactive molecule in enhancing plant tolerance to paraquat-induced oxidative stress via the modulation of heme oxygenase-1 signalling system. Plant Cell Environ 36:956–969
Melis A, Happe T (2001) Hydrogen production. Green algae as a source of energy. Plant Physiol 127:740–748
Melis A, Melnicki MR (2006) Integrated biological hydrogen production. Int J Hydrog Energy 31:1563–1573
Ohsawa I, Ishikawa M, Takahashi K, Watanabe M, Nishimaki K, Yamagata K, Katsura K, Katayama Y, Asoh S, Ohta S (2007) Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat Med 13:688–694
Renwick GM, Giumarro C, Siegel SM (1964) Hydrogen metabolism in higher plants. Plant Physiol 39:303–306
Ryu MY, Cho SK, Kim WT (2010) The Arabidopsis C3H2C3-type RING E3 ubiquitin ligase AtAIRP1 is a positive regulator of an abscisic acid-dependent response to drought stress. Plant Physiol 154:1983–1997
Schachtman DP, Goodger JQ (2008) Chemical root to shoot signaling under drought. Trends Plant Sci 13:281–287
Slovik S, Daeter W, Hartung W (1995) Compartmental redistribution and long-distance transport of abscisic acid (ABA) in plants as influenced by environmental changes in the rhizosphere-a biomathematical model. J Exp Bot 46:881–894
Thordal-Christensen H, Zhang Z, Wei Y, Collinge DB (1997) Subcellular localization of H2O2 in plants. H2O2 accumulation in papillae and hypersensitive response during the barley-powdery mildew interaction. Plant J 11:1187–1194
Villiers F, Kwak JM (2013) Rapid apoplastic pH measurement in Arabidopsis leaves using a fluorescent dye. Plant Signal Behav 8:e22587
Wilkinson S, Davies WJ (2002) ABA-based chemical signalling: the co-ordination of responses to stress in plants. Plant Cell Environ 25:195–210
Xie Y, Mao Y, Lai D, Zhang W, Shen W (2012) H2 enhances Arabidopsis salt tolerance by manipulating ZAT10/12-mediated antioxidant defence and controlling sodium exclusion. PLoS One 7:e49800
Xie Y, Mao Y, Zhang W, Lai D, Wang Q, Shen W (2014) Reactive oxygen species-dependent nitric oxide production contributes to hydrogen-promoted stomatal closure in Arabidopsis. Plant Physiol 165:759–773
Zeng J, Zhang M, Sun X (2013) Molecular hydrogen is involved in phytohormone signaling and stress responses in plants. PLoS One 8:e71038
Acknowledgements
This research was supported by the National Natural Science Foundation of China (31371546 and J1210056), the Fundamental Research Funds for the Central Universities (KYTZ201402), and the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig. 1
Phenotypes of alfalfa seedlings pretreated with HRW in response to ABA during seed germination and early seedling growth (TIFF 4596 kb)
Supplementary Fig. 2
Fluorescence intensity of 8-hydroxypyrene-1,3,6-trisulfonic acid, trisodium salt (HTPS) as a function of pH (TIFF 190 kb)
Supplementary Fig. 3
A model detailing how H2 might be involved in the ABA-signal transduction network leading stomatal closure (TIFF 694 kb)
Rights and permissions
About this article
Cite this article
Jin, Q., Zhu, K., Cui, W. et al. Hydrogen-Modulated Stomatal Sensitivity to Abscisic Acid and Drought Tolerance Via the Regulation of Apoplastic pH in Medicago sativa . J Plant Growth Regul 35, 565–573 (2016). https://doi.org/10.1007/s00344-015-9561-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-015-9561-2