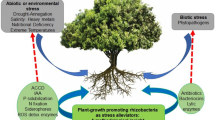
Abstract
The functional analysis of phytohormone production, interaction, and regulation in higher plants has re-emerged in the past 10 years due to spectacular advances in integrative study models. However, plants are not axenic in natural conditions and are usually colonized or influenced directly by different microorganisms such as rhizobacteria of which many have the ability to produce phytohormones. This review summarizes information related to the biosynthesis, metabolism, regulation, physiological role, and agronomical impact of phytohormones produced by the model plant-growth-promoting rhizobacteria (PGPR) belonging to the genus Azospirillum, considered to be one of the most representative PGPR. We include exhaustive information about the phytohormones auxins, gibberellins, cytokinins, ethylene, and abscisic acid, as well as the plant growth regulators polyamines and nitric oxide. We deal with their metabolism by Azospirillum sp. in chemically defined medium, in plant–microbe interactions, or in the context of the agronomical use of Azospirillum sp.





Similar content being viewed by others
References
Arshad M, Frankenberger R Jr (1993) Microbial production of plant growth regulators. In: Meeting B (ed) Soil microbial ecology. Marcel Dekker, New York, pp 307–347
Aziz A, Martin-Tanguy J, Larher F (1997) Plasticity of polyamine metabolism associated with high osmotic stress in rape leaf discs and with ethylene treatment. Plant Growth Regul 21:153–163
Baca B, Soto-Urzua L, Xochiua-Corona Y, Cuervo-García A (1994) Characterization of two aromatic amino acid aminotransferases and production of indoleacetic acid in Azospirillum strains. Soil Biol Biochem 26:57–63
Baldani V, Alvarez M, Baldani J, Döbereiner J (1986) Establishment of inoculated Azospirillum spp. in the rhizosphere and in roots of field grown wheat and sorghum. Plant Soil 90:35–46
Bally R, Thomas-Bauzon D, Heulin T, Balandreau J (1983) Determination of the most frequent N2 fixing bacteria in the rice rhizosphere. Can J Microbiol 29:881–887
Bar T, Okon Y (1993) Tryptophan conversion to indole-3-acetic acid via indole-3-acetamide in Azospirillum brasilense Sp7. Can J Microbiol 39:81–86
Barbieri P, Bernardi A, Galli E, Zanetti G (1988) Effects of inoculation with different strains of A. brasilense on wheat roots development. In: Klingmüller W (ed) Azospirillum IV. Genetics, physiology, ecology. Springer, Berlin, pp 181–188
Barea J, Navarro M, Montoya E (1976) Production of plant growth regulators by rhizosphere phosphate-solubilizing bacteria. J Appl Bacteriol 40:129–134
Bashan Y, de Bashan L (2010) How the plant growth-promoting bacterium Azospirillum promotes plant growth: a critical assessment. Adv Agron 108:77–136
Bashan Y, Holguín G (1998) Proposal for the division of plant growth-promoting rhizobacteria into two classifications: biocontrol-PGPB (plant growth promoting bacteria) and PGPB. Soil Biol Biochem 30:1225–1228
Bashan Y, Levanony H (1990) Current status of Azospirillum inoculation technology: Azospirillum as a challenge for agriculture. Can J Microbiol 36:591–608
Baudoin E, Lerner A, Mirza MS, El Zemrany H, Prigent-Combaret C, Jurkevich E, Spaepen S, Vanderleyden J, Nazaret S, Okon Y et al (2010) Effects of Azospirillum brasilense with genetically modified auxin biosynthesis gene ipdC upon the diversity of the indigenous microbiota of the wheat rhizosphere. Res Microbiol 161:219–226
Bergersen F (1971) Biochemistry of symbiotic nitrogen fixation in legumes. Ann Rev Plant Physiol 22:121–140
Blaha D, Prigent-Combaret C, Mirza M, Möenne-Loccoz Y (2006) Phylogeny of the 1-aminocyclopropane-1-carboxylic acid deaminase-encoding gene acdS in phytobeneficial and pathogenic Proteobacteria and relation with strain biogeography. FEMS Microbiol Ecol 56:455–470
Bothe H, Körsgen H, Lehmaher T, Hundeshagen B (1992) Differential effects of Azospirillum, auxin and combined nitrogen on the growth of the roots of wheat. Symbiosis 13:167–179
Bottini R, Fulchieri M, Pearce D, Pharis R (1989) Identification of gibberellins A1, A3, and Iso-A3 in cultures of A. lipoferum. Plant Physiol 90:45–47
Bottini R, Cassán F, Piccoli P (2004) Gibberellin production by bacteria and its involvement in plant growth promotion and yield increase. Appl Microbiol Biotechnol 65(5):497–503
Burdman S, Volpin H, Kigel J, Kapulnik Y, Okon Y (1996) Promotion of nod gene inducers and nodulation in common bean (Phaseolus vulgaris) roots inoculated with Azospirillum brasilense Cd. Appl Environ Microbiol 62:3030–3033
Burg S (1962) The physiology of ethylene formation. Ann Rev Plant Physiol 13:265–302
Burg S, Burg P (1968) Ethylene formation in pea seedlings: its relation to the inhibition of bud growth caused by indole-3-acetic acid. Plant Physiol 43:1069–1073
Cacciari I, Lippi D, Pietrosanti T (1989) Phytohormone-like substances produced by single and mixed diazotrophic cultures of Azospirillum spp. and Arthrobacter. Plant Soil 115:151–153
Carreño-López R, Campos-Reales C, Elmerich C, Baca B (2000) Physiological evidence for differently regulated tryptophan-dependent pathways for indole-3-acetic acid synthesis in Azospirillum brasilense. Mol Gen Genet 264:521–530
Cassán F, Bottini R, Piccoli P (2001a) In vivo gibberellin A9 metabolism by Azospirillum sp. in dy dwarf rice mutants seedlings. PGRSA Q 28:124–129
Cassán F, Bottini R, Schneider G, Piccoli P (2001b) Azospirillum brasilense and Azospirillum lipoferum hydrolize conjugates of GA20 and metabolize the resultant aglycones to GA1 in seedlings of rice dwarf mutants. Plant Physiol 125:2053–2058
Cassán F, Lucangelli C, Bottini R, Piccoli P (2001c) Azospirillum spp. metabolize [17,17-2H2]gibberellin A20 to [17,17-2H2] gibberellin A1 in vivo in dy rice mutant seedlings. Plant Cell Physiol 42:763–767
Cassán F, Maiale S, Masciarelli O, Vidal A, Luna V, Ruiz O (2009a) Cadaverine production by Azospirillum brasilense and its possible role in plant growth promotion and osmotic stress mitigation. Eur J Soil Biol 45:12–19
Cassán F, Perrig D, Sgroy V, Masciarelli O, Penna C, Luna V (2009b) Azospirillum brasilense Az39 and Bradyrhizobium japonicum E 109 promote seed germination and early seedling growth, independently or co-inoculated in maize (Zea mays L.) and soybean (Glycine max L.). Eur J Soil Biol 45:28–35
Castro-Guerrero J, Romero A, Aguilar J, Xiqui M, Sandoval J, Baca B (2012) The hisC1 gene, encoding aromatic amino acid aminotransferase-1 in Azospirillum brasilense Sp7, expressed in wheat. Plant Soil 356:139–150
Charyulu P, Fourcassie F, Barbouche A, Rondro Harisoa L, Omar A, Weinhard P, Marie R, Balandreau J (1985) Field inoculation of rice using in vitro selected bacterial and plant genotypes. In: Klingmüller W (ed) Azospirillum III: genetics, physiology, ecology. Springer, Berlin, pp 163–179
Cohen A, Bottini R, Piccoli P (2008) Azospirillum brasilense Sp 245 produces ABA in chemically-defined culture medium and increases ABA content in Arabidopsis plants. Plant Growth Regul 54:97–103
Cohen A, Travaglia C, Bottini R, Piccoli P (2009) Participation of abscisic acid and gibberellins produced by endophytic Azospirillum in the alleviation of drought effects in maize. Botany 87:455–462
Combes-Meynet E, Pothier JF, Moënne-Loccoz Y, Prigent-Combaret C (2011) The Pseudomonas secondary metabolite 2,4-diacetylphloroglucinol is a signal inducing rhizoplane expression of Azospirillum genes involved in plant-growth promotion. Mol Plant Microbe Interact 24:271–284
Conney T, Nonhebel H (1991) Biosynthesis of indole-3-acetic acid in tomato shoots: measurement, mass-spectral identification and incorporation of 2H from 2H2O into indole-3-acetic acid, d- and l-tryptophan, indole-3-pyruvate and tryptamine. Planta 184:368–376
Cooper J (2007) Early interactions between legumes and rhizobia: disclosing complexity in a molecular dialogue. J Appl Microbiol 103:1355–1365
Correa-Aragunde N, Graziano M, Chevalier C, Lamattina L (2006) Nitric oxide modulates the expression of cell cycle regulatory genes during lateral root formation in tomato. J Exp Bot 57:581–588
Costacurta A, Keijers V, Vanderleyden J (1994) Molecular cloning and sequence analysis of an Azospirillum brasilense indole-3-pyruvate deccarboxylase. Mol Gen Genet 243:463–472
Creus C, Graziano M, Casanovas E, Pereyra A, Simontacchi M, Puntarulo S, Barassi C, Lamattina L (2005) Nitric oxide is involved in the Azospirillum brasilense-induced lateral root formation in tomato. Planta 221:297–303
Crozier A, Arruda P, Jasmim JM, Monteiro AM, Sandberg G (1988) Analysis of indole-3-acetic acid and related indoles in culture medium from Azospirillum lipoferum and Azospirillum brasilense. Appl Environ Microbiol 54:2833–2837
Dardanelli M, Fernandez de Cordoba F, Espuny M, Rodriguez Carvajal M, Soria Diaz M, Gil Serrano A, Okon Y, Megias M (2008) Effect of Azospirillum brasilense coinoculated with Rhizobium on Phaseolus vulgaris flavonoids and Nod factor production under salt stress. Soil Biol Biochem 40:2713–2721
Davies P (1995) Plant hormones. physiology, biochemistry and molecular biology. Kluwer Academic, Dordrecht, p 833
Díaz-Zorita M, Fernández Canigia M (2009) Field performance of a liquid formulation of Azospirillum brasilense on dryland wheat productivity. Eur J Soil Biol 45(1):3–11
Dobbelaere S, Croonenborghs A, Thys A, Vande Broek A, Vanderleyden J (1999) Phytostimulatory effect of Azospirillum brasilense wild type and mutant strains altered in IAA production on wheat. Plant Soil 212:155–164
Esquivel-Cote R, Ramírez-Gama R, Tsuzuki-Reyes G, Orozco-Segovia A, Huante P (2010) Azospirillum lipoferum strain AZm5 containing 1-aminocyclopropane-1-carboxylic acid deaminase improves early growth of tomato seedlings under nitrogen deficiency. Plant Soil 337:65–75
Falik E, Okon Y, Epstein E, Goldman A, Fischer M (1989) Identification and quantification of IAA and IBA in Azospirillum brasilense-inoculated maize roots. Soil Biol Biochem 21:147–153
Ford Y, Taylor J, Blake P, Marks P (2002) Gibberellin A3 stimulates adventitious rooting of cuttings from cherry (Prunus avium). Plant Growth Regul 37:127–133
Frankenberger W, Arshad M (1995) Phytohormones in soil. Marcel Dekker, New York, p 503
Fulchieri M, Lucangelli C, Bottini R (1993) Inoculation with A. lipoferum affects growth and gibberellin status of corn seedlings roots. Plant Cell Physiol 34:1305–1309
Gamarnik A, Frydman R (1991) Cadaverine, an essential diamine for the normal root development of germinating soybean (Glycine max) seeds. Plant Physiol 97:778–785
Ge SM, Chen SF (2009) Expression and functional analysis of aminotransferase involved in indole-3-acetic acid biosynthesis in Azospirillum brasilense Yu62. Biochemistry (Moscow) 74(1):81–84
Glick B, Karaturovic D, Newell P (1995) A novel procedure for rapid isolation of plant growth promoting pseudomonads. Can J Microbiol 41:533–536
Glick B, Patten C, Holguin G, Penrose D (1999) Biochemical and genetic mechanisms used by plant growth promoting bacteria. Imperial College Press, London, p 267
Goris J, Kersters K, De Vos P (1998) Polyamines distribution among authentic Pseudomonads and Azotobacteraceae. Syst Appl Microbiol 21:285–290
Hamana K, Matsuzaki K, Sakakibara M (1988) Distribution of sym-homospermidine in eubacteria, cyanobacteria, algae and ferns. FEMS Microbiol Lett 50:11–16
Hamana K, Minamisawa K, Matsuzaki S (1990) Polyamines in Rhizobium, Bradyrhizobium, Azorhizobium and Agrobacterium. FEMS Microbiol Lett 71:71–76
Hartmann A, Singh M, Klingmüller W (1983) Isolation and characterization of Azospirillum mutants excreting high amounts of indoleacetic acid. Can J Microbiol 29:916–923
Hartmann A, Rothballer M, Schmid M (2008) Lorenz Hiltner, a pioneer in rhizosphere microbial ecology and soil bacteriology research. Plant Soil 312:7–14
Hedden P, Phillips AL (2000) Gibberellin metabolism: new insights revealed by the genes. Trends Plant Sci 5:523–530
Holguín G, Glick BR (2001) Expression of the ACC deaminase gene from Enterobacter cloacae UW4 in Azospirillum brasilense. Microb Ecol 41:281–288
Horemans S, Koninck K, Neuray J, Hermans R, Vlassak K (1986) Production of plant growth substances by Azospirillum sp. and other rhizophere bacteria. Symbiosis 2:341–346
Hubbell D, Tien T, Gaskins M, Lee J (1979) Physiological interaction in the Azospirillum-grass root association. In: Vose P, Ruschel A (eds) Associative N2-fixation. CRC Press, Boca Raton, pp 1–6
Inada S, Shimmen T (2000) Regulation of elongation growth by gibberellin in root segments of Lemna minor. Plant Cell Physiol 41:932–939
Janzen R, Rood S, Dormaar J, McGill W (1992) Azospirillum brasilense produces gibberellins in pure culture on chemically-defined medium and in co-culture on straw. Soil Biol Biochem 24:1061–1064
Kaneko T, Minamisawa K, Isawa T, Nakatsukasa H, Mitsui H et al (2010) Complete genomic structure of the cultivated rice endophyte Azospirillum sp. B510. DNA Res 17:37–50
Klee H, Montoya A, Horodyski F, Lichenstein C, Garfinkel D, Fuller S, Flores C, Peschon J, Nester E, Gordon M (1984) Nucleotide sequence of the tms genes of the pTiANC octopine C plasmid: two genes products involved in plants tumorogenesis. P Natl Acad Sci USA 81:1728–1732
Kloepper J, Schroth M (1978) Plant growth-promoting rhizobacteria in radish. In Proceedings of the 4th international conference on plant pathogenic bacteria, vol 2. Angers, INRA, France, pp 879-882
Kloepper J, Lifshitz R, Schroth M (1989) Pseudomonas inoculants to benefit plant production. ISI Atlas Sci Anim Plant Sci 8:60–64
Kobayashi M, Sakurai A, Saka A, Takahashi N (1989) Quantitative analysis of endogenous gibberellins in normal and dwarf cultivars of rice. Plant Cell Physiol 30:963–969
Kolb W, Martin P (1985) Response of plant roots to inoculation with Azospirillum brasilense and to application of indoleacetic acid. In: Klingmüller W (ed) Azospirillum III: genetics, physiology, ecology. Springer, Berlin, pp 215–221
Korasick DA, Enders TA, Strader LC (2013) Auxin biosynthesis and storage forms. J Exp Bot 64:2541–2555
Krumpholz E, Ribaudo C, Cassán F, Bottini R, Cantore M, Curá A (2006) Azospirillum sp. promotes root hair development in tomato plants through a mechanism that involves ethylene. J Plant Growth Regul 25:175–185
Kucey R (1988) Alteration of size of wheat root systems and nitrogen fixation by associative nitrogen-fixing bacteria measured under field conditions. Can J Microbiol 34:735–739
Kuznetsov V, Radyukina N, Shevyakova N (2006) Polyamines and stress: biological role, metabolism, and regulation. Russ J Plant Physiol 53:583–604
Lamattina L, Polacco J (2007) Nitric oxide in plant growth development and stress physiology. Springer, Berlin, p 283
Lambrecht M, Vande Broek A, Dosselaere F, Vanderleyden J (1999) The ipdC promoter auxin-responsive element of Azospirillum brasilense, a prokaryotic ancestral form of the plant AusxRE? Mol Microbiol 32:889–890
Lambrecht M, Okon Y, Vande Broek A, Vanderleyden J (2000) Indole-3-acetic acid: a reciprocal signalling molecule in bacteria-plant interactions. Trends Microbiol 8:298–300
Letham D (1963) Zeatin, a factor inducing cell division from Zea mays. Life Sci 8:569–573
Liu K, Fu H, Bei Q, Luan S (2000) Inward potassium channel in guard cells as a target for polyamine regulation of stomatal movements. Plant Physiol 124:1315–1325
Lucangelli C, Bottini R (1997) Effects of Azospirillum spp. on endogenous gibberellin content and growth of maize (Zea mays L.) treated with uniconazole. Symbiosis 23:63–72
Magalhães F, Baldani J, Souto S, Kuykendall J, Döbereiner J (1983) A new acid-tolerant Azospirillum species. An Acad Bras Ciênc 55:417–430
Malhotra M, Srivastava S (2008) Organization of the ipdC region regulates IAA levels in different Azospirillum brasilense strains: molecular and functional analysis of ipdC in strain SM. Environ Microbiol 10:1365–1373
Malhotra M, Srivastava S (2009) Stress-responsive indole-3-acetic acid biosynthesis by Azospirillum brasilense SM and its ability to modulate plant growth. Eur J Soil Biol 45:73–80
Martínez-Morales L, Soto-Urzua L, Baca B, Sanchez-Ahedo J (2003) Indole-3-butyric acid (IBA) production in culture medium by wild strain Azospirillum brasilense. FEMS Microbiol Lett 228:167–173
Mathesius U, Shalaman H, Meijer D, Lugtenberg B, Spaink H, Weinman J, Rodam L, Sautter C, Rolfe B, Djordjevic M (1997) New tools for investigating nodule initiation and ontogeny: spot inoculation and microtargeting of transgenic withe clover roots shows auxin involvement and suggest a role for flavonoids. In: Stacey G, Mullin B, Gresshoff P (eds) Advances in molecular genetics of plant–microbe interactions. Kluwer Academic, Dordrecht
Miller C, Skoog F, Von Saltza M, Strong F (1955) Kinetin, a cell division factor from deoxyribonucleic acid. J Am Chem Soc 77:1392
Molina-Favero C, Creus C, Lanteri M, Correa-Aragunde N, Lombardo M, Barassi C, Lamattina L (2007) Nitric oxide and plant growth promoting rhizobacteria: common features influencing root growth and development. Adv Bot Res 46:1–33
Molina-Favero C, Creus C, Simontacchi M, Puntarulo S, Lamattina L (2008) Aerobic nitric oxide production by Azospirillum brasilense Sp245 and its influence on root architecture in tomato. Mol Plant Microbe Interact 21:1001–1009
Murakami Y (1968) A new rice seedling bioassay for gibberellins, microdrop method and its use for testing extracts of rice and morning glory. Bot Mag 81:3–43
Murakami Y (1972) Dwarfing genes in rice and their relation to gibberellin biosynthesis. In: Carr D (ed) Plant growth substances 1970. Springer, Berlin, pp 164–174
Muralidhara R, Rai P (1986) Plant growth regulators produced by diazotrophic bacteria. National seminar on microbial ecology, January 23-24, 1986, Tamil Nadu Agricultural University, Tamil Nadu, India, pp 18-23
Nambara E, Marion-Poll A (2005) Abscisic acid biosynthesis and catabolism. Annu Rev Plant Biol 56:165–185
Niemi K, Haggman H, Sarjala T (2001) Effects of exogenous diamines on the interaction between ectomycorrhizal fungi and adventitious root formation in Scots pine in vitro. Tree Physiol 22:373–381
Nonhebel H, Cooney T, Simpson R (1993) The route, control and compartmentation of auxin synthesis. Aust J Plant Physiol 20:527–539
Okon Y, Labandera-González C (1994) Agronomic applications of Azospirillum: an evaluation of 20 years worlwide field inoculation. Soil Biol Biochem 26:1591–1601
Omay S, Schmidt W, Martin P, Bangerth F (1993) Indoleacetic acid production by the rhizosphere bacterium Azospirillum brasilense Cd under in vitro conditions. Can J Microbiol 39:187–192
Ona O, Smets I, Gysegom P, Bernaerts K, Impe J, Prinsen E, Vanderleyden J (2003) The effect of pH on indole-3-acetic acid (IAA) biosynthesis of Azospirillum brasilense sp7. Symbiosis 35:199–208
Ona O, van Impe J, Prinsen E, Vanderleyden J (2005) Growth and indole-3-acetic acid biosynthesis of Azospirillum brasilense Sp245 is environmentally controlled. FEMS Microbiol Lett 246:125–132
Patten C, Glick B (1996) Bacterial biosynthesis of indole 3-acetic acid. Can J Microbiol 42:207–220
Pearce D, Koshioka M, Pharis R (1994) Chromatography of gibberellins. J Chromatogr A 658:91–122
Pedraza R, Ramirez-Mata A, Xiqui M, Baca B (2004) Aromatic amino acid aminotransferase activity and indole-3-acetic acid production by associative nitrogen-fixing bacteria. FEMS Microbiol Lett 233:15–21
Perley J, Stowe B (1966) On the ability of Taphrina deformans to produce indole acetic acid from tryptophan by way of tryptamine. Plant Physiol 41:234–237
Perrig D, Boiero L, Masciarelli O, Penna C, Cassán F, Luna V (2007) Plant growth promoting compounds produced by two agronomically important strains of Azospirillum brasilense, and their implications for inoculant formulation. Appl Microbiol Biotechnol 75:1143–1150
Phinney B, Spray C (1988) Dwarf mutants of maize-research tools for the analysis of growth. In: Pharis R, Rood S (eds) Plant growth substances 1988. Springer, Berlin, pp 65–73
Piccoli P, Bottini R (1994a) Metabolism of 17,17-[2H2]-gibberellin A20 to 17,17-[2H2]-gibberellin A1 by A. lipoferum cultures. AgriScientiae 11:13–15
Piccoli P, Bottini R (1994b) Effect of C/N ratio, N content, pH and incubation time on growth and gibberellin production by Azospirillum lipoferum cultures. Symbiosis 21:263–264
Piccoli P, Bottini R (1996) Gibberellins production in A. lipoferum cultures and enhanced by light. Biocell 20:185–190
Piccoli P, Lucangelli C, Schneider G, Bottini R (1997) Hydrolisis of 17,17-[2H2]-gibberellin A20-glucoside and 17,17-[2H2]-gibberellin A20-glucosyl ester by Azospirillum lipoferum cultured in nitrogen-free biotin-based chemycally-definded medium. Plant Growth Regul 23:179–182
Piccoli P, Masciarelli O, Bottini R (1999) Gibberellin production by Azospirillum lipoferum cultured in chemically-defined medium as affected by oxygen availability and water status. Symbiosis 27:135–146
Piotrowski M (2008) Primary or secondary? Versatile nitrilases in plant metabolism. Phytochemistry 69:2655–2667
Primrose S, Dilworth M (1976) Ethylene production by bacteria. J Gen Microbiol 93:177–181
Prinsen E, Costacurta A, Michiels K, Vanderleyden J, Van Onckelen H (1993) Azospirillum brasilense indole-3-acetic acid biosynthesis: evidence for a non-tryptophan dependent pathway. Mol Plant Microbe Interact 6:609–615
Rademacher W (2000) Growth retardants: effects on gibberellin biosynthesis and other metabolic pathways. Ann Rev Plant Physiol Plant Mol Biol 51:501–531
Rahman A, Hosokawa S, Oono Y, Amakawa T, Goto N, Tsurumi S (2002) Auxin and ethylene response interactions during Arabidopsis root hair development diss. Plant Physiol 130:1908–1917
Remans R, Beebe S, Blair M, Marique G, Tovar E, Rao I, Croonenbarghs A, Torres-Gutierrez R, El-Idoweity M, Michiels J, Vanderlyden J (2008a) Physiological and genetic analysis of root responsiveness to auxin-producing plant growth promoting bacteria in common bean (Phaseolus vulgaris L.). Plant Soil 302:149–161
Remans R, Schelkens S, Hernandez G, Garcia A, Luis Reyes J, Mendez N, Toscano V, Mulling M, Galvez L, Vanderleyden J (2008b) Effect of Rihzobium–Azospirillum coinoculation on nitrogen fixation and yield of two contrasting Phaseolus vulgaris L. genotypes cultivated across different environments in cube. Plant Soil 312:25–37
Rodrigues E, Rodrigues L, de Oliveira A, Baldani V, Teixeira K, Urquiaga S, Reis V (2008) Azospirillum amazonense inoculation: effects on growth, yield and N2 fixation of rice (Oryza sativa L.). Plant Soil 302:249–261
Rood S, Pharis R (1987) Evidence for reversible conjugation of gibberellins in higher plants. In: Schreiber H, Schutte H, Semder G (eds), Conjugated plant hormones. Structure, metabolism and function. In Proceedings of the international symposium conjugated plant hormones: structure, metabolism and function held in Gera, Germany. Berlin, VEB Deustcher Verlag der Wissenschaften, pp 183-190
Ross J, O’Neill D (2001) New interactions between classical plant hormones. Trends Plant Sci 6:2–4
Rothballer M, Schmid M, Fekete A, Hartmann A (2005) Comparative in situ analysis of ipdC-gfpmut3 promoter fusions of Azospirillum brasilense Sp7 and Sp245. Environ Microbiol 7:1839–1846
Ruckäschel E, Klingmüller W (1992) Analysis of IAA biosynthesis in Azospirillum lipoferum and Tn5 induced mutants. Symbiosis 13:123–131
Sakakibara H (2006) Cytokinins: activity, biosynthesis, and translocation. Annu Rev Plant Biol 57:431–449
Sant’Anna F, Almeida L, Cecagno R, Reolon L, Siqueira F, Machado M, Vasconcelos A, Schrank I (2011) Genomic insights into the versatility of the plant growth-promoting bacterium Azospirillum amazonense. BMC Genomics 12:409
Schmidt W, Martin P, Omay H, Bangerth F (1988) Influence of Azospirillum brasilense on nodulation of legumes. In: Klingmüller W (ed) Azospirillim IV. Genetics, physiology, ecology. Springer, Heidelberg, pp 92–100
Sembder G, Gross D, Liebisch H, Schneider G (1980) Biosynthesis and metabolism of plant hormones. In: MacMillan J (ed) Encyclopedia of plant physiology, new series. Springer, Berlin, pp 281–444
Somers E, Ptacek D, Gysegom P, Srinivasan M, Vanderleyden J (2005) Azospirillum brasilense produces the auxin-like phenylacetic acid by using the key enzyme for indole-3-acetic acid biosynthesis. Appl Environ Microb 71:1803–1810
Spaepen S, Vanderleyden J, Remans R (2007) Indole-3-acetic acid in microbial and microorganism-plant signaling. FEMS Microbiol Rev 31:425–448
Spaepen S, Dobbelaere S, Croonenborghs A, Vanderleyden J (2008) Effects of Azospirillum brasilense indole-3-acetic acid production on inoculated wheat plants. Plant Soil 312:15–23
Strzelczyk E, Kamper M, Li C (1994) Cytokinin-like substances and ethylene production by Azospirillum in media with different carbon sources. Microbiol Res 149:55–60
Teale W, Paponov I, Palme K (2006) Auxin in action: signalling, transport and the control of plant growth and development. Nat Rev Mol Cell Biol 7:847–859
Thiman K (1936) On the physiology of the formation of nodule in legumes roots. Proc Natl Acad Sci USA 22:511–514
Thuler D, Floh E, Handro W, Barbosa H (2003a) Beijerinckia derxii releases plant growth regulators and amino acids in synthetic media independent of nitrogenase activity. J Appl Microbiol 95:799–806
Thuler D, Floh E, Handro W, Barbosa H (2003b) Plant growth regulators and amino acids released by Azospirillum sp. in chemically defined media. Lett Appl Microbiol 37:174–178
Tien T, Gaskins M, Hubbell D (1979) Plant growth substances produced by Azsopirillum brasilense and their effect on the growth of pearl millet (Pennisetum americanum L.). Appl Environ Microbiol 37:1016–1024
Van Laer S (2003) PhD thesis, University of Antwerpen, Belgium
Van Puyvelde S, Cloots L, Engelen K, Das F, Marchal K, Vanderleyden J, Spaepen S (2011) Transcriptome analysis of the rhizosphere bacterium Azospirillum brasilense reveals an extensive auxin response. Microb Ecol 61:723–728
Vande Broek A, Lambrecht M, Eggermont K, Vanderleyden J (1999) Auxins upregulate expression of the indole-3-pyruvate decarboxylase gene in Azospirillum brasilense. J Bacteriol 181:1338–1342
Vande Broek A, Gysegom P, Ona O, Hendrickx N, Prinsen E, Van Impe J, Vanderleyden J (2005) Transcriptional analysis of the Azospirillum brasilense indole-3-pyruvate decarboxylase gene and identification of a cis-acting sequence involved in auxin responsive expression. Mol Plant Microbe Interact 18(4):311–323
Vorwerk S, Biernacki S, Hillebrand H, Janzik I, Muller A, Weiler E, Piotrowski M (2001) Enzymatic characterization of the recombinant Arabidopsis thaliana nitrilase subfamily encoded by the NIT2/NIT1/NIT3-gene cluster. Planta 212:508–516
Wisniewski-Dyé F, Borziak K, Khalsa-Moyers G, Alexandre G, Sukharnikov L et al (2011) Azospirillum genomes reveal transition of bacteria from aquatic to terrestrial environments. PLoS Genet 7(12):e1002430
Wisniewski-Dyé F, Lozano L, Acosta-Cruz E, Borland S, Drogue B, Prigent-Combaret et al (2012) Genome sequence of Azospirillum brasilense CBG497 and comparative analyses of Azospirillum core and accessory genomes provide insight into niche adaptation. Genes 3:576–602
Yahalom E, Okon Y, Dovrat A (1990) Possible mode of action of Azospirillum brasilense strain Cd on the roots morphology and nodule formation in burr medic (Medicago polymorpha). Can J Microbiol 36:10–14
Yaxley J, Ross J, Sherriff L, Reid J (2001) Gibberellin biosynthesis mutations and root development in pea. Plant Physiol 125:627–633
Zeevaart J (1999) Abscisic acid metabolism and its regulation. In: Hooykaas P, Hall M, Libbenga K (eds) Biochemistry and molecular biology of plant hormones. Elsevier Science, Amsterdam, pp 189–207
Zhu JK (2002) Salt and drought stress signal transduction in plants. Annu Rev Plant Biol 53:247–273
Zimmer W, Roeben K, Bothe H (1988) An alternative explanation for plant growth promotion by bacteria of the genus Azospirillum. Planta 176:333–342
Acknowledgments
The review was written in the framework of a bilateral FWO-Vlaanderen-MINCyT research project (VS.011.11N) granted to FC and JV. FC is a researcher of Consejo Nacional de Investigaciones Científico-Tecnológicas (CONICET) and Universidad Nacional de Río Cuarto (UNRC) and SS is a recipient of a postdoctoral fellowship grant from Research Foundation Flanders (FWO-Vlaanderen). Special thanks to Yoav Bashan (CBNOR) and Cecilia Creus (INTA-UNMdP) for providing information to complete the phytohormonal model of Azospirillum sp.
Conflict of interest
The authors have no conflict of interest to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cassán, F., Vanderleyden, J. & Spaepen, S. Physiological and Agronomical Aspects of Phytohormone Production by Model Plant-Growth-Promoting Rhizobacteria (PGPR) Belonging to the Genus Azospirillum . J Plant Growth Regul 33, 440–459 (2014). https://doi.org/10.1007/s00344-013-9362-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-013-9362-4