Abstract
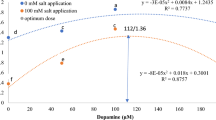
We investigated the effects of the secondary metabolite protocatechualdehyde (PCA, 3,4-dihydroxybenzaldehyde) on stress markers, including fluorescence parameters and the concentrations of pigments, free radicals, protein, and lipid peroxides, in adult plants of Arabidopsis thaliana. The content of proline, carotenoids, and chlorophylls a and b peaked 9 h after administration of 3 mM PCA (the highest concentration used in this study), although malonyldialdehyde and dry mass contents peaked 24 h following PCA treatment. Leaf staining revealed peak production of O2 − between 30 and 90 min post-treatment and peak production of H2O2 between 1 and 9 h post-treatment. Several effects, including the observed furling of leaf margins (leaf rolling), the increases in proline content and dry to fresh weight ratio, and the oxidative burst, are reminiscent of the plant response to drought. Early dehydration in PCA-treated plants was confirmed by decreases in leaf water potential, relative water content, and stomatal opening in the first hours of treatment. Thus, PCA seems to be either inducing water deficiency stress (probably through action in the roots) or directly triggering antidrought defenses. In either case, plants showed tolerance to the concentrations employed in this study, with most of the parameters observed having recovered control values within a week of treatment.









Similar content being viewed by others
References
Bates L, Waldren RP, Teare ID (1973) Rapid determination of free proline for water stress studies. Plant Soil 39:205–207
Begg JE (1980) Morphological adaptation of leaves to water stress. In: Turner NC, Kramer PJ (eds) Adaptation of plants to water and high temperature stress. Wiley Interscience, New York, pp 33–42
Bright J, Desikan R, Hancock JT, Weir IS, Neill SJ (2006) ABA-induced NO generation and stomatal closure in Arabidopsis are dependent on H2O2 synthesis. Plant J 45:113–122
Chempakam B, Kasturi Hai KV, Rajagopal V (1993) Lipid peroxidation in relation to drought tolerance in coconut (Cocos nucijera L.). Plant Physiol Biochem 20:5–11
Clarke JM (1986) Effect of leaf rolling on leaf water loss in Triticum spp. Can J Plant Sci 66:885–891
Deuner S, Alves JD, Zanandrea I, Pereira Goulart P, Silveira NM, de Castro Henrique P, Mesquita AC (2011) Stomatal behavior and components of the antioxidative system in coffee plants under water stress. Sci Agric 68:77–85
Duke SO, Rimando A, Scheffler B, Dayan FE (2002) Strategies for research in applied aspects of allelopathy. Plymouth. In: Reigosa MJ, Pedrol N (eds) Allelopathy: from molecules to ecosystems. Science Publishers, UK, pp 139–152
Gershenzon J (2002) Secondary metabolism and plant defense. In: Taiz L, Zeiger E (eds) Plant physiology, 3rd edn. Sinauer Associates, Sunderland, MA, pp 283–308
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930
Gonzáles WL, Suárez LH, Molina-Montenegroa MA, Gianolia E (2008) Water availability limits tolerance of apical damage in the Chilean tarweed Madia sativa. Acta Oecol 34:104–110
González L (2001) Determination of water potential in leaves. In: Reigosa MJ (ed) Handbook of plant ecophysiology techniques. Kluwer, Dordrecht, pp 193–206
González L, Reigosa MJ (2001) Plant water status. In: Reigosa MJ (ed) Handbook of plant ecophysiology techniques. Kluwer, Dordrecht, pp 185–192
Gzik A (1996) Accumulation of proline and pattern of α-amino acids in sugar beet plants in response to osmotic, water and salt stress. Environ Exp Bot 36:29–38
Hadaçek F (2002) Secondary metabolites as plant traits: Current assessment and future perspectives. Crit Rev Plant Sci 21:273–322
Halliwell B, Gutteridge JMC (1985) The chemistry of oxygen radicals and other oxygen-derived species. In: Free radicals in biology and medicine. Oxford University Press, New York, pp 20–64
Hoagland DR, Arnon DI (1950) The water culture method for growing plants without soil. Calif Agric Exp Station, Berkeley, CA, Circ No 347, p 142
Hodges DM, DeLong JM, Forney CF, Prange RK (1999) Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 207:604–611
Hsiao TC, O’Toole JC, Yambao EB, Turner N (1984) Influence of osmotic adjustment on leaf rolling and tissue death in rice (Oryza sativa L.). Plant Physiol 75:338–341
Hsu YCh, Lin YL, Chiu YT, Shiao MS, Lee ChY, Huang YT (2005) Antifibrotic effects of Salvia miltiorrhiza on dimethylnitrosamine-intoxicated rats. J Biomed Sci 12:185–195
Huang XY, Chao DY, Gao JP, Zhu MZ, Shi M, Lin HX (2009) A previously unknown zinc finger protein, DST, regulates drought and salt tolerance in rice via stomatal aperture control. Genes Dev 23:1805–1817
Kadioglu A, Terzi R (2007) A dehydration avoidance mechanism: leaf rolling. Bot Rev 73:290–302
Kadioglu A, Turgut R (1999) Some biochemical changes during leaf rolling in Ctenanthe setosa (Marantaceae). Acta Physiol Plant 21:209–214
Kim KJ, Kim MA, Jung JH (2008) Antitumor and antioxidant activity of protocatechualdehyde produced from Streptomyces lincolnensis M-20. Arch Pharmacal Res 31:1572–1577
Kliebenstein DJ (2004) Secondary metabolites and plant/environment interactions: a view through Arabidopsis thaliana tinged glasses. Plant Cell Environ 27:675–684
Klughammer C, Schreiber U (2008) Complementary PS II quantum yields calculated from simple fluorescence parameters measured by PAM fluorometry and the Saturation Pulse method. PAM Appl Notes 1:27–35
Kramer DM, Johnson G, Kiirats O, Edwards GE (2004) New fluorescence parameters for the determination of QA redox states and excitation energy fluxes. Photos Res 79:209–218
Kraus TE, McKersie BD, Fletcher RA (1995) Paclobutrazol-induced tolerance of wheat leaves to paraquat may involve increased antioxidant enzyme activity. J Plant Physiol 145:570–576
Lu Q, Wen X, Lu C, Zhang Q, Kuang T (2003) Photoinhibition and photoprotection in senescent leaves of field-grown wheat plants. Plant Physiol Biochem 41:749–754
Macías FA (1995) Allelopathy in the search for natural herbicide models. ACS Symp Ser 582:310–329
Macías FA, Molinillo MJG, Castellano D, Velasco RF (1999) Sesquiterpene lactones with potential use as natural herbicide models (I): trans, trans-Germacranolide. J Agric Food Chem 47:4407–4414
Martínez-Peñalver A, Reigosa MJ, Sánchez-Moreiras AM (2011) Imaging chlorophyll a fluorescence reveals specific spatial distributions under different stress condition. Flora 206:836–844
McAinsh MR, Clayton H, Mansfield TA, Hetherington AM (1996) Changes in stomatal behavior and guard cell cytosolic free calcium in response to oxidative stress. Plant Physiol 111:1031–1042
Meister MH, Bolhàr-Nordenkampf HB (2001) Stomata imprints. A new and quick method to count stomata and epidermic cells. In: Reigosa MJ (ed) Handbook of plant ecophysiology techniques. Kluwer, Dordrecht, pp 235–250
Neill SJ, Desikan R, Clarke A, Hurst RD, Hancock JT (2002) Hydrogen peroxide and nitric oxide as signalling molecules in plants. J Exp Bot 53:1237–1247
O’Toole JC, Cruz RT, Singh TN (1979) Leaf rolling and transpiration. Plant Sci Lett 16:111–114
Oxborough K, Baker NR (1997) Resolving chlorophyll a fluorescence images of photosynthetic efficiency into photochemical and non-photochemical components—calculation of qP and F’v/F’m without measuring F’o. Photosynth Res 54:135–142
Pedrol N, Ramos P (2001) Protein content quantification by Bradford method. In: Reigosa MJ (ed) Handbook of plant ecophysiology techniques. Kluwer, Dordrecht, pp 283–296
Pei ZM, Murata Y, Benning G, Thomine S, Klusener B, Allen GJ, Grill E, Schroeder JI (2000) Calcium channels activated by hydrogen peroxide mediate abscisic acid signaling in guard cells. Nature 406:731–734
Reigosa MJ, Pazos-Malvido E (2007) Phytotoxic effects of 21 plant secondary metabolites on Arabidopsis thaliana germination and root growth. J Chem Ecol 33:1456–1466
Sánchez-Moreiras AM, Martínez-Peñalver A, Reigosa MJ (2011) Early senescence induced by 2-3H-benzoxazolinone (BOA) in Arabidopsis thaliana. J Plant Physiol 168:863–870
Scholander PF, Hammel HT, Bradstreet ED, Hemmingsen EA (1965) Sap pressure in vascular plants. Science 148:339–346
Singh TN LG, Paleg LG, Aspinall D (1973) Stress metabolism. III. Variations in response to water deficit in the barley plant. Austral J Biol Sci 26:65–76
Sivaramakrishnan S, Patell VZ, Flower DJ, Peacock JM (1988) Proline accumulation and nitrate reductase activity in contrasting sorghum lines during mid-season drought stress. Physiol Plant 74:418–426
Slatyer RO (1967) Plant-water relationships. Academic Press, London
Unsicker SB, Kunert G, Gershenzon J (2009) Protective perfumes: the role of vegetative volatiles in plant defense against herbivores. Curr Opin Plant Biol 12:479–485
Van Acker FAA, Schouten O, Haenen GRMM, Van der Vijgh WJF, Bast A (2000) Flavonoids can replace tocopherol as an antioxidant. FEBS Letters 473:145–148
Wang S, Gao Z, Li E, Su C, Zhu H (2008) The application with protocatechualdehyde to improve anticoagulant activity and cell affinity of silk fibroin. Appl Surf Sci 225:486–488
Weatherley PE (1950) Studies in the water relations of the cotton plant. I. The field measurement of water deficit in leaves. New Phytol 49:81–97
Weber B, Hoesch L, Rast DM (1995) Protocatechualdehyde and other phenols as cell wall components of grapevine leaves. Phytochemistry 40:433–437
Wellburn AR (1994) The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J Plant Physiol 144:307–313
Wink M (2010) Introduction: biochemistry, physiology and ecological functions of secondary metabolites. In: Wink M (ed) Biochemistry of plant secondary metabolism, 2nd edn. Wiley-Blackwell, Oxford, pp 1–19
Yin Y, Li S, Liao W, Lu Q, Wen X, Lu C (2010) Photosystem II photochemistry, photoinhibition, and the xanthophyll cycle in heat-stressed rice leaves. J Plant Physiol 167:959–966
Yu K, Wang YW, Cheng YY (2006) Determination of protocatechuic aldehyde, danshensu, salvianolic acid B and gallic acid in chinese medicine ‘SUANGDAN’ granule by MEKC. Chromatographia 63:393–398
Zhang J, Kirkham MB (1996) Enzymatic responses of the ascorbate-glutathione cycle to drought in sorghum and sunflower plants. Plant Sci 113:139–147
Zhang X, Zhang L, Dong F, Gao J, Galbraith DW, Song CP (2001) Hydrogen peroxide is involved in abscisic acid-induced stomatal closure in Vicia faba. Plant Physiol 126:1438–1448
Zhang AL, Ye Q, Li BG, Qi HY, Zhang GL (2005) Phenolic and triterpene glycosides from the stems of Ilex litseaefolia. J Nat Prod 68:1531–1535
Zhou Z, Zhang Y, Ding XR, Chen SH, Yang J, Wang XJ, Jia GL, Chen HS, XCh Bo, Wang SQ (2006) Protocatechuic aldehyde inhibits hepatitis B virus replication both in vitro and in vivo. Antivir Res 74:59–64
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Martínez-Peñalver, A., Pedrol, N., Reigosa, M.J. et al. Tolerance of Arabidopsis thaliana to the Allelochemical Protocatechualdehyde. J Plant Growth Regul 31, 406–415 (2012). https://doi.org/10.1007/s00344-011-9250-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-011-9250-8