Abstract
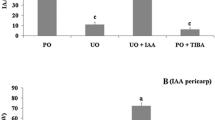
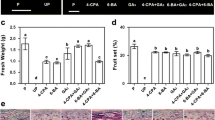
The effect of applied gibberellin (GA) and auxin on fruit-set and growth has been investigated in tomato (Solanum lycopersicum L.) cv Micro-Tom. It was found that to prevent competition between developing fruits only one fruit per truss should be left on the plant. Unpollinated ovaries responded to GA3 and to different auxins [indol-3-acetic acid, naphthaleneacetic acid, and 2,4-dichlorophenoxyacetic acid (2,4-D)], 2,4-D being the most efficient. GA3- and 2,4-D-induced fruits had different internal morphology, with poor locular tissue development in the case of GA, and pseudoembryos development in the case of 2,4-D. Also, GA3 produced larger cells in the internal region of the mesocarp (IM) associated with higher mean C values, whereas 2,4-D produced more cell layers in the pericarp than pollinated fruits. The smaller size of GA3- compared with 2,4-D-induced fruits was due to them having fewer cells, only partially compensated by the larger size of IM cells. Simultaneous application of GA3 and 2,4-D produced parthenocarpic fruits similar to pollinated fruits, but for the absence of seeds, suggesting that both kinds of hormones are involved in the induction of fruit development upon pollination. It is concluded that Micro-Tom constitutes a convenient model system, compared to tall cultivars, to investigate the hormonal regulation of fruit development in tomato.








Similar content being viewed by others
References
Abad M, Monteiro AA (1989) The use of auxins for the production of greenhouse tomatoes in mild-winter conditions: a review. Sci Hort 38:167–192
Alabadí D, Carbonell J (1998) Expression of ornithine decarboxylase is transiently increased by pollination, 2,4-dichlorophenoxyacetic acid, and gibberellic acid in tomato ovaries. Plant Physiol 118:323–328
Alabadí D, Agüero MS, Pérez-Amador MA, Carbonell J (1996) Arginase, arginine decarboxylase, ornithine decarboxylase, and polyamines in tomato ovaries. Changes in pollinated ovaries and parthenocarpic fruits induced by auxin and gibberellin. Plant Physiol 112:1237–1244
Asahira T, Takeda Y, Nishio T, Hirabayashi M, Tsukamoto Y (1967) Studies on fruit development in tomato. I. Ovule development and content of diffusible auxin in synthetic auxin- and gibberellin-induced parthenocarpic tomato fruits in relation to their development. Mem Res Inst Food Sci Kyoto Univ 28:47–74
Asahira T, Takagi H, Takeda Y, Tsukamoto Y (1968) Studies on fruit development on tomato. II. Cytokinin activity in extracts from pollinated, auxin- and gibberellin-induced parthenocarpic tomato fruits and its effect on the histology of the fruit. Mem Res Inst Food Sci Kyoto Univ 29:24–54
Barow M, Meister A (2003) Endopolyploidy in seed plants is differently correlated to systematics, organ, life strategy and genome size. Plant Cell Environ 26:571–584
Bergervoet JHW, Verhoeven HA, Gilissen LJW, Bino RJ (1996) High amounts of nuclear DNA in tomato (Lycopersicon esculentum Mill.) pericarp. Plant Sci 116:141–145
Bertin N, Borel C, Brunel B, Cheniclet C, Causse M (2003) Do genetic make-up and growth manipulation affect tomato fruit size by cell number, or cell size and DNA endoreduplication? Ann Bot 92:415–424
Bohner J, Bangerth F (1988) Cell number, cell size and hormone levels in semi-isogenic mutants of Lycopersicon pimpinellifolium differing in fruit size. Physiol Plant 72:316–320
Bünger-Kibler S, Bangerth F (1982/83) Relationship between cell number, cell size and fruit size of seeded fruits of tomato (Lycopersicon esculentum Mill.), and those induced parthenocarpically by the application of plant growth regulators. Plant Growth Regul 1:143–154
Cheniclet C, Rong WY, Causse M, Frangne N, Bolling L, Carde JP, Renaudin JP (2005) Cell expansion and endoreduplication show a large genetic variability in pericarp and contribute strongly to tomato fruit growth. Plant Physiol 139:1984–1994
Cowling RJ, Harberd NP (1999) Gibberellins control Arabidopsis hypocotyl growth via regulation of cellular elongation. J Exp Bot 50:1351–1357
Dan Y, Yan H, Munyikwa T, Dong J, Zhang Y, Armstrong CL (2006) MicroTom, a high-throughput model transformation system for functional genomics. Plant Cell Rep 25:432–441
Eyal E, Levy AA (2002) Tomato mutants as tools for functional genomics. Curr Opin Plant Biol 5:112–117
Fos M, Nuez F, García-Martínez JL (2000) The gene pat-2, which induces natural parthenocarpy, alters the gibberellin content in unpollinated tomato ovaries. Plant Physiol 122:471–479
Fos M, Proaño K, Nuez F, García-Martínez JL (2001) Role of gibberellins in parthenocarpic fruit development induced by the genetic system pat-3/pat-4 in tomato. Physiol Plant 111:545–550
Gillaspy G, Ben-David H, Gruissem W (1993) Fruits: a developmental perspective. Plant Cell 5:1439–1451
Gorquet B, Van Heusden AW, Lindhout P (2005) Parthenocarpic fruit development in tomato. Plant Biol 7:131–139
Ho L, Hewitt J (1986) Fruit development. In: Atherton JG, Rudish J (eds), The tomato crop. New York: Chapman and Hall, pp 201–239
Joubès J, Phan T-H, Just D. Rothan C. Bergounioux C, Raymond P, Chevalier C (1999) Molecular and biochemical characterization of the involvement of cyclin-dependent kinase A during the early development of tomato fruit. Plant Physiol 121:857–869
Kataoka K, Uemachi A, Yazawa S (2003) Fruit growth and pseudoembryo development affected by uniconazole, an inhibitor of gibberellins, in pat-2 and auxin-induced parthenocarpic tomato fruits. Sci Hort 98:9–16
Koshioka M, Nishijima T, Yamazaki H, Liu Y, Nonaka M, Mander LN (1994) Analysis of gibberellins in growing fruits of Lycopersicon esculentum after pollination or treatment with 4-chlorophenoxyacetic acid. J Hort Sci 69:171–179
Lemaire-Chamley M, Petit J, García V, Just D, Baldet P, Germain V, Fagard M, Mouassite M, Cheniclet C, Rothan C (2005) Changes in transcriptional profiles are associated with early fruit tissue specialization in tomato. Plant Physiol 139:750–769
Mapelli SC, Frova C, Torti G, Soressi G (1978) Relationship between set, development and activities of growth regulators in tomato fruits. Plant Cell Physiol 19:1281–1288
Martí E, Gisbert C, Bishop GJ, Dixon MS, García-Martínez JL (2006) Genetic and physiological characterization of tomato cv. Micro-Tom. J Exp Bot 57:2037–2047
Meissner R, Jacobson Y, Melamed S, Levyatuv S, Shalev G, Ashri A, Elkind Y, Levy AA (1997) A new model system for tomato genetics. Plant J 12:1465–1472
Montoya T, Nomura T, Yokota T, Farrar K, Harrison K, Jones JGD, Kaneta T, Kamiya Y, Szekeres M, Bishop G (2005) Patterns of Dwarf expression and brassinosteroid accumulation in tomato reveal the importance of brassinosteroid synthesis during fruit development. Plant J 42:262–269
Pandolfini T, Rotino GL, Camerini S, Defez R, Spena A (2002) Optimisation of transgene action at the post-transcriptional level: high quality parthenocarpic fruits in industrial tomatoes. BMC Biotechnol 2:1–11
Scott JW, Harbaugh BK (1989) Micro-Tom. - A miniature dwarf tomato. Florida Agric Exp Sta Cir 370:1–6
Sjut V, Bangerth F (1981) Effect of pollination or treatment with growth regulators on levels of extractable hormones in tomato ovaries and young fruits. Physiol Plant 53:76–78
Sjut V, Bangerth F (1982/1983) Induced parthenocarpy – a way of changing the levels of endogenous hormones in tomato fruits (Lycopersicon esculentum Mill.). 1 Extractable hormones. Plant Growth Regul 1:243–251
Srivastava A, Handa AK (2005) Hormonal regulation of tomato fruit development: a molecular perspective. J Plant Growth Regul 24:67–82
Tsukaya H (2006) Mechanism of leaf-shape determination. Ann Rev Plant Biol 57:477–496
Vercher Y, Carbonell J (1991) Changes in the structure of ovary tissues and in the ultrastructure of mesocarp cells during ovary senescence or fruit development induced by plant growth substances in Pisum sativum. Physiol Plant 81:518–526
Wang H, Jones B, Li Z, Frasse P, Delalande C, Regad F, Chaabouni S, Latché A, Pech J-C, Bouzayen M (2005) The tomato Aux/IAA transcription factor IAA9 is involved in fruit development and leaf morphogenesis. Plant Cell 17:2676–2692
Acknowledgments
The authors thank Dr. A. Levy for providing the tomato cv Micro-Tom seeds, Dr. A. Granell for comments to the manuscript, and Dr. M. D. Gómez for microscopy support. The work was supported by Ministerio de Educación y Ciencia, Spain (grant project BIO2003-00151).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Serrani, J.C., Fos, M., Atarés, A. et al. Effect of Gibberellin and Auxin on Parthenocarpic Fruit Growth Induction in the cv Micro-Tom of Tomato. J Plant Growth Regul 26, 211–221 (2007). https://doi.org/10.1007/s00344-007-9014-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-007-9014-7