Abstract
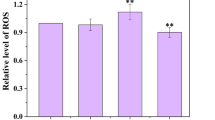
Copper sulfate (CuSO4) is widely used in controlling the Microcystis aeruginosa blooms. Many studies reported the toxicity mechanisms of Cu2+ to M. aeruginosa at the physiological level, but little is known about a transcriptomic basis of these mechanisms. In the present study, M. aeruginosa was treated by 0.5 mg/L Cu2+ (half of the 96-h EC50) for 72 h. The results show that Cu2+ content in M. aeruginosa increased after 72 h Cu2+ exposure, whereas the Fv/Fm chlorophyll fluorescence value and chlorophyll a content in M. aeruginosa sharply decreased. Reactive oxygen species concentration and activity of antioxidant enzymes (superoxide dismutase, catalase and peroxidase) were all increased. These physiological data confirmed toxicity of Cu2+ to M. aeruginosa. The RNA-seq analysis showed that 6 646 725 and 7 880 291 clean reads were obtained for the Cu-treated and control libraries, respectively. The 595 genes (252 downward trend and 343 upward trend) with the Gene Ontology (GO) annotations were divided into three main functional categories: cellular component, molecular function, and biological process. In the Cluster of Orthologous Groups (COG) annotation, 418 differentially expressed genes with 25 functional definitions were obtained. Among them, ‘replication, recombination and repair’, ‘energy production and conversion’, and ‘general function prediction only’ were the largest three groups of transcripts. In the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis, genes involved in photosynthesis and oxidative phosphorylation were present at the highest percentages. In addition, the genes involved in photosynthesis and oxidative phosphorylation were identified, and then confirmed using real-time PCR. This study reported the first transcriptome of M. aeruginosa. Photosynthesis and oxidative phosphorylation were severely affected by Cu2+ toxicity, which may have contributed to cell death. These data provide the potential mechanism to explain the CuSO4 effect on the harmful M. aeruginosa.
Similar content being viewed by others
References
Anders S, Huber W. 2010. Differential expression analysis for sequence count data. Genome Biology, 11(10): R106.
Baker N R. 2008. Chlorophyll fluorescence: a probe of photosynthesis in vivo. Annual Review of Plant Biology, 59: 89–113.
Barrington D J, Ghadouani A. 2008. Application of hydrogen peroxide for the removal of toxic cyanobacteria and other phytoplankton from wastewater. Environmental Science & Technology, 42(23): 8 916–8 921.
Boucher N, Carpentier R. 1999. Hg2+, Cu2+, and Pb2+-induced changes in photosystem II photochemical yield and energy storage in isolated thylakoid membranes: a study using simultaneous fluorescence and photoacoustic measurements. Photosynthesis Research, 59(2–3): 167–174.
Bradford M M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72(1–2): 248–254.
Chen L, Mao F J, Kirumba G C, Jiang C, Manefield M, He Y L. 2015. Changes in metabolites, antioxidant system, and gene expression in Microcystis aeruginosa under sodium chloride stress. Ecotoxicology and Environmental Safety, 122: 126–135.
Conesa A, Götz S, García-Gómez J M, Terol J, Talón M, Robles M. 2005. Blast2GO: a universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics, 21(18): 3 674–3 676.
Foster S, Thomson D, Maher W. 2008. Uptake and metabolism of arsenate by anexic cultures of the microalgae Dunaliella tertiolecta and Phaeodactylum tricornutum. Marine Chemistry, 108(3–4): 172–183.
Gai S P, Zhang Y X, Mu P, Liu C Y, Liu S, Dong L, Zheng G S. 2012. Transcriptome analysis of tree peony during chilling requirement fulfillment: assembling, annotation and markers discovering. Gene, 497(2): 256–262.
Garcia-Molina A, Andrés-Colás N, Perea-García A, Del Valle-Tascón S, Peñarrubia L, Puig S. 2011. The intracellular Arabidopsis COPT5 transport protein is required for photosynthetic electron transport under severe copper deficiency. Plant Journal, 65(6): 848–860.
Gera A, Alcoverro T, Mascaró O, Pérez M, Romero J. 2012. Exploring the utility of Posidonia oceanica chlorophyll fluorescence as an indicator of water quality within the European Water Framework Directive. Environmental Monitoring and Assessment, 184(6): 3 675–3 686.
Gui D, Liu W B, Shao X P, Xu W N. 2010. Effects of different dietary levels of cottonseed meal protein hydrolysate on growth, digestibility, body composition and serum biochemical indices in crucian carp (Carassius auratus gibelio). Animal Feed Science and Technology, 156(3–4): 112–120.
Guo R Y, Lim W A, Ki J S. 2016a. Genome-wide analysis of transcription and photosynthesis inhibition in the harmful dinoflagellate Prorocentrum minimum in response to the biocide copper sulfate. Harmful Algae, 57: 27–38.
Guo R Y, Wang H, Suh Y S, Ki J S. 2016b. Transcriptomic profiles reveal the genome-wide responses of the harmful dinoflagellate Cochlodinium polykrikoides when exposed to the algicide copper sulfate. BMC Genomics, 17: 29.
Hadjoudja S, Vignoles C, Deluchat V, Lenain JF, Le Jeune AH, Baudu M. 2009. Short term copper toxicity on Microcystis aeruginosa and Chlorella vulgaris using flow cytometry. Aquatic Toxicology, 94(4): 255–264.
Hanawalt P C. 2007. Paradigms for the Three Rs: DNA replication, recombination, and repair. Molecular Cell, 28(5): 702–707.
Hong Y, Hu H Y, Xie X, Li F M. 2008. Responses of enzymatic antioxidants and non-enzymatic antioxidants in the cyanobacterium Microcystis aeruginosa to the allelochemical ethyl 2-methyl acetoacetate (EMA) isolated from reed (Phragmites communis). Journal of Plant Physiology, 165(12): 1 264–1 273.
Hrudey S, Burch M D, Drikas M, Gregory R. 1999. Remedial measures. In: Chorus I, Bartram J eds. Toxic Cyanobacteria in Water: A Guide to Their Public Health Consequences, Monitoring, and Management. E & FN Spon, New York. p.275–312.
Kanehisa M, Goto S. 2000. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Research, 28(1): 27–30.
Kaneko T, Nakajima N, Okamoto S, Suzuki I, Tanabe Y, Tamaoki M, Nakamura Y, Kasai F, Watanabe A, Kawashima K, Kishida Y, Ono A, Shimizu Y, Takahashi C, Minami C, Fujishiro T, Kohara M, Katoh M, Nakazaki N, Nakayama S, Yamada M, Tabata S, Watanabe MM. 2007. Complete genomic structure of the bloom-forming toxic cyanobacterium Microcystis aeruginosa NIES-843. DNA Research, 14(6): 247–256.
Kuno S, Bacher A, Simon H. 1985. Structure of enoate reductase from a Clostridium tyrobutyricum (C. spec. La1). Biological Chemistry Hoppe-Seyler, 366(5): 463–472.
Leng X P, Jia H F, Sun X, Shangguan L F, Mu Q, Wang B J, Fang J G. 2015. Comparative transcriptome analysis of grapevine in response to copper stress. Scientific Reports, 5: 17749.
Leu E, Krieger-Liszkay A, Goussias C, Gross E M. 2002. Polyphenolic allelochemicals from the aquatic angiosperm Myriophyllum spicatum inhibit photosystem II. Plant Physiology, 130(4): 2 011–2 018.
Lu L, Wu Y X, Ding H J, Zhang W H. 2015. The combined and second exposure effect of copper (II) and chlortetracycline on fresh water algae, Chlorella pyrenoidosa and Microcystis aeruginosa. Environmental Toxicology and Pharmacology, 40(1): 140–148.
Lu Y P, Wang J, Yu Y, Shi L M, Kong F X. 2014. Changes in the physiology and gene expression of Microcystis aeruginosa under EGCG stress. Chemosphere, 117: 164–169.
Mulo P, Sakurai I, Aro E M. 2012. Strategies for psbA gene expression in cyanobacteria, green algae and higher plants: from transcription to PSII repair. Biochimica et Biophysica Acta, 1817(1): 247–257.
Ohkawa H, Ohishi N, Yagi K. 1979. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Analytical Biochemistry, 95(2): 351–358.
Otten T G, Paerl H W. 2015. Health effects of toxic cyanobacteria in U.S. drinking and recreational waters: our current understanding and proposed direction. Current Environmental Health Reports, 2(1): 75–84.
Powell M A, Somero G N. 1986. Hydrogen sulfide oxidation is coupled to oxidative phosphorylation in mitochondria of Solemya reidi. Science, 233(4763): 563–566.
Rascioa N, Navari-Izzo F. 2011. Heavy metal hyperaccumulating plants: how and why do they do it? And what makes them so interesting? Plant Science, 180(2): 169–181.
Shao J H, Xu Y, Wang Z J, Jiang Y G, Yu G L, Peng X, Li R H. 2011. Elucidating the toxicity targets of β-ionone on photosynthetic system of Microcystis aeruginosa NIES-843 (Cyanobacteria). Aquatic Toxicology, 104(1–2): 48–55.
Trapnell C, Williams B A, Pertea G, Mortazavi A, Kwan G, van Baren M J, Salzberg S L, Wold B J, Pachter L. 2010. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nature Biotechnology, 28(5): 511–515.
Tsai K P. 2015. Effects of two copper compounds on Microcystis aeruginosa cell density, membrane integrity, and microcystin release. Ecotoxicology and Environmental Safety, 120: 428–435.
Wang T, Long X H, Chen X Y, Liu Y R, Liu Z P, Han S Q, Yan S H. 2017. Integrated transcriptome, proteome and physiology analysis of Epinephelus coioides after exposure to copper nanoparticles or copper sulfate. Nanotoxicology, 11(2): 236–246.
Wang X H, Hui F, Yang Y C, Yang S H. 2018. Deep sequencing and transcriptome analysis to identify genes related to biosynthesis of aristolochic acid in Asarum heterotropoides. Scientific Reports, 8: 17 850.
Wang Y J, Dong C L, Xue Z Y, Jin Q J, Xu Y C. 2016. De novo transcriptome sequencing and discovery of genes related to copper tolerance in Paeonia ostii. Gene, 576(1): 126–135.
Wu H M, Wei G J, Tan X, Li L, Li M. 2017. Species-dependent variation in sensitivity of Microcystis species to copper sulfate: implication in algal toxicity of copper and controls of blooms. Scientific Reports, 7: 40 393.
Wu H X, Jia H M, Ma X W, Wang S B, Yao Q S, Xu W T, Zhou Y G, Gao Z S, Zhan R L. 2014. Transcriptome and proteomic analysis of mango (Mangifera indica Linn) fruits. Journal of Proteomics, 105: 19–30.
Zhang S L, Xing K Z, Zhou Y. 2007. The acute toxicity of copper ion on alga Microcystis aeruginosa. Fisheries Science, 26(6): 323–326. (in Chinese with English abstract)
Zhou S Q, Shao Y S, Gao N Y, Deng Y, Qiao J L, Ou H S, Deng J. 2013. Effects of different algaecides on the photosynthetic capacity, cell integrity and microcystin-LR release of Microcystis aeruginosa. Science of the Total Environment, 463–464: 111–119.
Zou H X, Pang Q Y, Zhang A Q, Lin L D, Li N, Yan X F. 2015. Excess copper induced proteomic changes in the marine brown algae Sargassum fusiforme. Ecotoxicology and Environmental Safety, 111: 271–280.
Acknowledgement
We thank Genepioneer Biotechnologies (Nanjing, China) for the assistance in analysis of the RNA-seq data.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by the Natural Science Foundation of Jiangsu Province of China (No. BK20180728), the Key Research and Development Program of Jiangsu Province (No. BE2017377), and the Creation Project of Major New Species of Agriculture in Jiangsu Province (No. PZCZ201742)
Rights and permissions
About this article
Cite this article
Wang, T., Hu, Y., Zhu, M. et al. Integrated transcriptome and physiology analysis of Microcystis aeruginosa after exposure to copper sulfate. J. Ocean. Limnol. 38, 102–113 (2020). https://doi.org/10.1007/s00343-019-8357-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00343-019-8357-9