Abstract
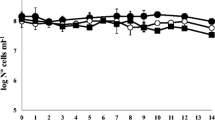
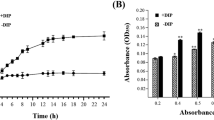
Vibrio harveyi is a pathogen of various aquatic organisms that has been recently associated with massive mortality episodes in the aquaculture industry. Recurrent outbreaks of vibriosis are closely correlated with the capacity of this bacterial species to survive long-term starvation conditions. To study the regulation mechanism of gene expression at the transcriptional level in V. harveyi under starvation conditions, the transcriptomic response profiles were determined of the Portunus trituberculatus pathogen V. harveyi strain DY1 under normal conditions and after four weeks of starvation. A total of 4 679 and 4 661 genes were expressed in the non-starved and starved cells, respectively. The significantly differentially expressed genes (DEGs) between non-starved and starved groups were identified, in which 255 genes were up-regulated and 411 genes were down-regulated. GO analysis and KEGG enrichment analysis were used to analyze the DEGs and revealed the involvement of these DEGs in many pathways, including ABC transporters, flagellum assembly, and fatty acid metabolism. Several DEGs were randomly selected and their expression levels were confirmed by quantitative real-time PCR (qRT-PCR). This is the first comprehensive transcriptomic analysis of starvation effects in V. harveyi. Our findings will facilitate future study on stress adaptation and survival mechanisms of V. harveyi.
Similar content being viewed by others
Data Availability Statement
All sequence data that support the findings of this study have been deposited in the NCBI Short Read. The sequence read archive (SRA) accession number: PRJNA507871.
References
Alcaide E, Gil-Sanz C, Sanjuán E, Esteve D, Amaro C, Silveira L. 2010. Vibrio harveyi causes disease in seahorse, Hippocampus sp. Journal of Fish Diseases, 24(5): 311–313.
Arias C R, LaFrentz S, Cai W L, Olivares-Fuster O. 2012. Adaptive response to starvation in the fish pathogen flavobacterium columnare: cell viability and ultrastructural changes. BMC Microbiology, 12: 266.
Austin B, Zhang X H. 2006. Vibrio harveyi: a significant pathogen of marine vertebrates and invertebrates. Letters in Applied Microbiology, 43(2): 119–124.
Austin B. 2010. Vibrios as causal agents of zoonoses. Veterinary Microbiology, 140(3–4): 310–317.
Biosca E G, Amaro C, Marco-Noales E, Oliver J D. 1996. Effect of low temperature on starvation-survival of the eel pathogen Vibrio vulnificus biotype 2. Applied and Environmental Microbiology, 62(2): 450–455.
Company R, Sitj-Bobadilla A, Pujalte M J, Garay E, Alvarez-Pellitero P. 1999. Bacterial and parasitic pathogens in cultured common dentex, Dentex dentex L. Journal of Fish Diseases, 22(4): 299–309.
Diggles B K, Moss G A, Carson J, Anderson C D. 2000. Luminous vibriosis in rock lobster Jasus verreauxi (Decapoda: Palinuridae) phyllosoma larvae associated with infection by Vibrio harveyi. Diseases of Aquatic Organisms, 43(2): 127–137.
Gauger E, Smolowitz R, Uhlinger K, Casey J, Gómez-Chiarri M. 2006. Vibrio harveyi and other bacterial pathogens in cultured summer flounder, Paralichthys dentatus. Aquaculture, 260(1–4): 10–20.
Haldar S, Maharajan A, Chatterjee S, Hunter S A, Chowdhury N, Hinenoya A, Asakura M, Yamasaki S. 2010. Identification of Vibrio harveyi as a causative bacterium for a tail rot disease of sea bream Sparus aurata from research hatchery in Malta. Microbiological Research, 165(8): 639–648.
Higgins C F. 2001. ABC transporters: physiology, structure and mechanism—an overview. Research in Microbiology, 152(3–4): 205–210.
Hispano C, Nebra Y, Blanch A R. 1997. Isolation of Vibrio harveyi from an ocular lesion in the short sunfish (Mola mola). Bulletin of the European Association of Fish Pathologists, 17(3–4): 104–107.
Jiang X, Chai T J. 1996. Survival of Vibrio parahaemolyticus at low temperatures under starvation conditions and subsequent resuscitation of viable, nonculturable cells. Applied and Environmental Microbiology, 62(4): 1 300–1 305.
Johnson F H, Shunk I V. 1936. An interesting new species of luminous bacteria. Journal of Bacteriology, 31(6): 585–593.
Kaberdin V R, Bläsi U. 2006. Translation initiation and the fate of bacterial mRNAs. FEMS Microbiology Reviews, 30(6): 967–979.
Kaberdin V R, Montánchez I, Parada C, Orruño M, Arana I, Barcina I. 2015. Unveiling the metabolic pathways associated with the adaptive reduction of cell size during vibrio harveyi persistence in seawater microcosms. Microbial Ecology, 70(3): 689–700.
Kim C, Mwangi M, Chung M, Milheirço C, De Lencastre H, Tomasz A. 2013. The mechanism of heterogeneous beta-lactam resistance in MRSA: key role of the stringent stress response. PLoS One, 8(12): e82814.
Kjellerberg S, Humphrey B A, Marshall K C. 1983. Initial phases of starvation and activity of bacteria at surfaces. Applied and Environmental Microbiology, 46(5): 978–984.
Kraxberger-Beatty T, McGarey D J, Grier H J, Lim D V. 1990. Vibrio harveyi, an opportunistic pathogen of common snook, Centropomus undecimalis (Bloch), held in captivity. Journal of Fish Diseases, 13(6): 557–560.
Kunttu H M T, Valtonen E T, Jokinen E I, Suomalainen L R. 2009. Saprophytism of a fish pathogen as a transmission strategy. Epidemics, 1(2): 96–100.
Lavilla-Pitogo C R, Baticados M C L, Cruz-Lacierda E R, de la Pena L D. 1990. Occurrence of luminous bacterial disease of Penaeus monodon larvae in the Philippines. Aquaculture, 91(1–2): 1–13.
Li R Q, Yu C, Li Y R, Lam T W, Yiu S M, Kristiansen K, Wang J. 2009. SOAP2: an improved ultrafast tool for short read alignment. Bioinformatics, 25(15): 1 966–1 967.
Liu P C, Lee K K, Yii K C, Kou G H, Chen S N. 1996. News & Notes: isolation of Vibrio harveyi from diseased kuruma prawns Penaeus japonicus. Current Microbiology, 33(2): 129–132.
McDougald D, Rice S A, Weichart D, Kjelleberg S. 1998. Nonculturability: adaptation or debilitation? FEMS Microbiology Ecology, 25(1): 1–9.
Montánchez I, Arana I, Parada C, Garaizabal I, Orruño M, Barcina I, Kaberdin V R. 2014. Reprogramming of Vibrio harveyi gene expression during adaptation in cold seawater. FEMS Microbiology Ecology, 87(1): 193–203.
Montánchez I, Ogayar E, Plágaro A H, Esteve-Codina A, Gómez-Garrido J, Orruño M, Arana I, Kaberdin V R. 2019. Analysis of Vibrio harveyi adaptation in sea water microcosms at elevated temperature provides insights into the putative mechanisms of its persistence and spread in the time of global warming. Scientific Reports, 9(1): 289.
Mortazavi A, Williams B A, McCue K, Schaeffer L, Wold B. 2008. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nature Methods, 5(7): 621–628.
Novitsky JA, Morita R Y. 1976. Morphological characterization of small cells resulting from nutrient starvation of a psychrophilic marine vibrio. Applied and Environmental Microbiolog, 32(4): 617–622.
Ortigosa M, Garay E, Pujalte M J. 1994. Numerical taxonomy of Vibrionaceae isolated from oysters and seawater along an annual cycle. Systematic and Applied Microbiology, 17(2): 216–225.
Parada C, Orruño M, Kaberdin V, Bravo Z, Barcina I, Arana I. 2016. Changes in the Vibrio harveyi cell envelope subproteome during permanence in cold seawater. Microbial Ecology, 72(3): 549–558.
Poindexter J S. 1981. Oligotrophy: fast and famine existence. In: Alexander M ed. Advances in Microbial Ecology. Springer, Boston, MA. p.63–89.
Pujalte M J, Sitjà-Bobadilla A, Macián M C, Belloch C, Álvarez-Pellitero P, Pérez-Sánchez J, Uruburu F, Garay E. 2003. Virulence and molecular typing of Vibrio harveyi strains isolated from cultured dentex, gilthead sea bream and European sea bass. Systematic and Applied Microbiology, 26(2): 284–292.
Saeed M O. 1995. Association of Vibrio harveyi with mortalities in cultured marine fish in Kuwait. Aquaculture, 136(1–2): 21–29.
Sun J J, Gao X J, Qun J, Du X D, Bi K R, Zhang X J, Lin L. 2016. Comparative analysis of the survival and gene expression of pathogenic strains Vibrio harveyi after starvation. FEMS Microbiology Letters, 363(22): fnw250, https://doi.org/10.1093/femsle/fnw250.
Suzina N E, Mulyukin A L, Kozlova A N, Shorokhova A P, Dmitriev V V, Barinova E S, Mokhova O N, El’-Registan G I, Duda V I. 2004. Ultrastructure of resting cells of some non-spore-forming bacteria. Microbiology, 73(4): 435–447.
Svensson S L, Pryjma M, Gaynor E C. 2014. Flagella-mediated adhesion and extracellular DNA release contribute to biofilm formation and stress tolerance of Campylobacter jejuni. PLoS One, 9(8): e106063.
Vatsos I N, Thompson K D, Adams A. 2003. Starvation of Flavobacterium psychrophilum in broth, stream water and distilled water. Diseases of Aquatic Organisms, 56(2): 115–126.
Wai S N, Mizunoe Y, Yoshida S I. 1999. How Vibrio cholerae survive during starvation. FEMS Microbiology Letters, 180(2): 123–131.
Wolf P W, Oliver J D. 1992. Temperature effects on the viable but non-culturable state of Vibrio vulnificus. FEMS Microbiology Letters, 101(1): 33–39.
Zhang X H, Austin B. 2000. Pathogenicity of Vibrio harveyi to salmonids. Journal of Fish Diseases, 23(2): 93–102.
Zhang X J, Bai X S, Yan B L, Bi K R, Qin L. 2014. Vibrio harveyi as a causative agent of mass mortalities of megalopa in the seed production of swimming crab Portunus trituberculatus. Aquaculture International, 22(2): 661–672
Zorrilla I, Arijo S, Chabrillon M, Diaz P, Martinez-Manzanares E, Balebona M C, Moriñigo M A. 2003. Vibrio species isolated from diseased farmed sole, Solea senegalensis (Kaup), and evaluation of the potential virulence role of their extracellular products. Journal of Fish Diseases, 26(2): 103–108.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by the Jiangsu Agricultural Industry Technology System (No. 02), the National Natural Science Foundation of China (No. 31972830), the Natural Science Foundation of Jiangsu Province (No. BK20180915), the Jiangsu Agriculture Science and Technology Innovation Fund (No. CX(18)2012), the Fishery Science and Technology Innovation Projects of Jiangsu Province (No. D2017-3), the “Blue Project” of Yangzhou University and the Projects of Shuichan Sanxin of Jiangsu Province (No. Y2016-33, D2017-3)
Rights and permissions
About this article
Cite this article
Liu, X., Gao, X., Chen, N. et al. Transcriptional responses to starvation of pathogenic Vibrio harveyi strain DY1. J. Ocean. Limnol. 38, 579–587 (2020). https://doi.org/10.1007/s00343-019-8350-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00343-019-8350-3