Abstract
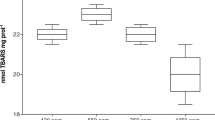
Although the impact of ocean acidification on marine bivalves has been previously investigated under mainly controlled laboratory conditions, it is still unclear whether the impact of acidification on sediment-burrowing species differs between those within or without sediment. In order to fill this gap in our knowledge, we compared shell properties of the infaunal Manila clam (Ruditapes philippinarum) exposed to three pH concentrations (7.4, 7.7, and 8.0), within and without sediments. In the first experiment (140 d), clams were exposed to seawater in an acidification system without sediment. A decrease in shell weight corresponding to the increase in dissolution rate was observed in the group of pH 7.4, at which shell color disappeared or whitened. SEM observations confirmed the changes of the external shell surface. In the second experiment (170 d), sediment was placed at the bottom of each exposure chamber. The effects were found obvious in shell dissolution rate and shell color in the shell specimens exposed to overlying seawater but not found in the shell specimens exposed to sediment. Although the experimental period was longer in the second experiment, shell specimens in the first experiment were more seriously damaged than those in the second experiment under acidic seawater conditions. Our results, in relation to the defense function of the shell, show that marine bivalves in burrowing behavior are more adaptable to seawater acidification than those who do not burrow into sediment.
Similar content being viewed by others
References
Albright R, Takeshita Y, Koweek D A, Ninokawa A, Wolfe K, Rivlin T, Nebuchina Y, Young J, Caldeira K. 2018. Carbon dioxide addition to coral reef waters suppresses net community calcification. Nature, 555(7697): 516–519.
Amaral V, Cabral H N, Bishop M J. 2012. Moderate acidification affects growth but not survival of 6-month-old oysters. Aquatic Ecology, 46(1): 119–127.
Beniash E, Ivanina A, Lieb N S, Kurochkin I, Sokolova I M. 2010. Elevated level of carbon dioxide affects metabolism and shell formation in oysters Crassostrea virginica Marine Ecology Progress Series, 419: 95–108.
Berge J A, Bjerkeng B, Pettersen O, Schaanning M T, Øxnevad S. 2006. effects of increased sea water concentrations of CO2 on growth of the bivalve Mytilus edulis L. Chemosphere, 62(4): 681–687.
Bibby R, Widdicombe S, Parry H, Spicer J, Pipe R. 2008. Effects of ocean acidification on the immune response of the blue mussel Mytilus edulis Aquatic Biology2(1): 67–74.
Bressan M, Chinellato A, Munari M, Matozzo V, Manci A, Marčeta T, Finos L, Moro I, Pastore P, Badocco D, Marin M G. 2014. Does seawater acidification affect survival, growth and shell integrity in bivalve juveniles? Marine Environmental Research, 99: 136–148.
Busch D S, Maher M, Thibodeau P, McElhany P. 2014. Shell condition and survival of Puget Sound pteropods are impaired by ocean acidification conditions. PLoS One9(8): e105884.
Caldeira K, Wickett M E. 2003. Anthropogenic carbon and ocean pH. Nature, 425(6956): 365.
Clements J C, Bourque D, McLaughlin J, Stephenson M, Comeau L A. 2017. Extreme ocean acidification reduces the susceptibility of eastern oyster shells to a polydorid parasite. Journal of Fish Diseases, 40(11): 1 573–1 585.
Clements J C, Coffin M R S, Lavaud R, Guyondet T, Comeau L. 2018. Ocean acidification and molluscan shell taphonomy: can elevated seawater p CO2 influence taphonomy in a naticid predator-prey system? Palaeogeography Palaeoclimatology Palaeoecology 507: 145–154.
Clements J C, Hunt H L. 2014. influence of sediment acidification and water flow on sediment acceptance and dispersal of juvenile soft-shell clams (Mya arenaria L.). Journal of Experimental Marine Biology and Ecology453: 62–69.
Clements J C, Hunt H L. 2018. Testing for sediment acidification effects on within-season variability in juvenile soft-shell clam (Mya arenaria) abundance on the northern shore of the Bay of Fundy. Estuaries and Coasts41(2): 471–483.
Cornwall C E, Hurd C L. 2016. Experimental design in ocean acidification research: problems and solutions. ICES Journal of Marine Science, 73(3): 572–581.
Cummings V, Hewitt J, Van Rooyen A, Currie K, Beard S, Thrush S, Norkko J, Barr N, Heath P, Halliday N J, Sedcole R, Gomez A, McGraw C, Metcalf V. 2011. Ocean acidification at high latitudes: potential effects on functioning of the Antarctic bivalve. Laternula elliptica PLoS One, 6(1): e16069.
Dickinson G H, Ivanina A V, Matoo O B, Pörtner H O, Lannig G, Bock C, Beniash E, Sokolova I M. 2012. Interactive effects of salinity and elevated CO2 levels on juvenile eastern oysters, Crassostrea virginica Journal of Experimental Biology, 215(1): 29–43.
Doney S C, Fabry V J, Feely R A, Kleypas J A. 2009. Ocean acidification: the other CO2 problem. Annual Review of Marine Science, 1: 169–192.
Duarte C, Navarro J M, Acuña K, Torres R, Manríquez P H, Lardies M A, Vargas C A, Lagos N A, Aguilera V. 2015. Intraspecific variability in the response of the edible mussel Mytilus chilensis (Hupe) to ocean acidification. Estuaries and Coasts, 38(2): 590–598.
Fabry V J, Seibel B A, Feely R A, Orr J C. 2008. Impacts of ocean acidification on marine fauna and ecosystem processes. ICES Journal of Marine Science65(3): 414–432.
Feely R A, Sabine C L, Lee K, Berelson W, Kleypas J, Fabry V J, Millero F J. 2004. Impact of anthropogenic CO2 on the CaCO 3 system in the oceans. Science305(5682): 362–366.
Fernández-Reiriz M J, Range P, Álvarez-Salgado X A, Espinosa J, Labarta U. 2012. Tolerance of juvenile Mytilus galloprovincialis to experimental seawater acidification. Marine Ecology Progress Series, 454: 65–74.
Gazeau F, Parker L M, Comeau S, Gattuso J P, O’Connor W A, Martin S, Pörtner H O, Ross P M. 2013. Impacts of ocean acidification on marine shelled molluscs. Marine Biology160(8): 2 207–2 245.
Gazeau F, van Rijswijk P, Pozzato L, Middelburg J J. 2014. Impacts of ocean acidification on sediment processes in shallow waters of the Arctic Ocean. PLoS One9(4): e94068.
Green M A, Jones M E, Boudreau C L, Moore R L, Westman B A. 2004. Dissolution mortality of juvenile bivalves in coastal marine deposits. Limnology and Oceanography49(3): 727–734.
Green M A, Waldbusse G G, Reilly S L, Emerson K, O’Donnell S. 2009. Death by dissolution: sediment saturation state as a mortality factor for juvenile bivalves. Limnology and Oceanography, 54(4): 1 037–1 047.
Gutiérrez J L, Jones C G, Strayer D L, Iribarne O O. 2003. Mollusks as ecosystem engineers: the role of shell production in aquatic habitats. Oikos, 101(1): 79–90.
Han K N, Lee S W, Wang S Y. 2008. The effect of temperature on the energy budget of the Manila clam, Ruditapes philippinarum Aquaculture International16(2): 143–152.
Hoegh-Guldberg O, Cai R, Poloczanska E S, Brewer P G, Sundby S, Hilmi K, Fabry V J, Jung S. 2014. The ocean. In: IPCC ed. Climate Chang 2014: Impacts, Adaptation, and Vulnerability. Part B: Regional Aspects. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge.
Houghton J T, Ding Y, Griggs D J, Noguer M, van der Linden P J, Dai X, Maskell K, Johnson C A. 2001. Climate Change 2001: the Scientific Basis. Cambridge University Press, Cambridge.
Jansson A, Norkko J, Norkko A. 2013. effects of reduced pH on Macoma balthica larvae from a system with naturally fluctuating pH-dynamics. PLoS One, 8(6): e68198.
Ji J, Choi H J, Ahn I Y. 2006. Evaluation of Manila clam Ruditapes philippinarum as a sentinel species for metal pollution monitoring in estuarine tidal flats of Korea: effects of size, sex, and spawning on baseline accumulation. Marine Pollution Bulletin52(4): 447–453.
Kitidis V, Laverock B, McNeill L C, Beesley A, Cummings D, Tait K, Osborn M A, Widdicombe S. 2011. Impact of ocean acidification on benthic and water column ammonia oxidation. Geophysical Research Letters38(21): L21603.
Le Moullac G, Soyez C, Vidal-Dupiol J, Belliard C, Fievet J, Sham-Koua M, Lo-Yat A, Saulnier D, Gaertner-Mazouni N, Gueguen Y. 2016. Impact of p CO2 on the energy, reproduction and growth of the shell of the pearl oyster Pinctada margaritifera. Estuarine Coastal and Shelf Science, 182: 274–282.
Liu W G, He M X. 2012. effects of ocean acidification on the metabolic rates of three species of bivalve from southern coast of China. Chinese Journal of Oceanology and Limnology, 30(2): 206–211.
McClintock J B, Angus R A, Mcdonald M R, Amsler C D, Catledge S A, Vohra Y K. 2009. Rapid dissolution of shells of weakly calcified Antarctic benthic macroorganisms indicates high vulnerability to ocean acidification. Antarctic Science, 21(5): 449–456.
Navarro J M, Torres R, Acuña K, Duarte C, Manriquez P H, Lardies M, Lagos N A, Vargas C, Aguilera V. 2013. Impact of medium-term exposure to elevated p CO2 levels on the physiological energetics of the mussel Mytilus chilensis. Chemosphere, 90(3): 1 242–1 248.
Onitsuka T, Takami H, Muraoka D, Matsumoto Y, Nakatsubo A, Kimura R, Ono T, Nojiri Y. 2018. effects of ocean acidification with p CO2 diurnal fluctuations on survival and larval shell formation of Ezo abalone, Haliotis discus hannai. Marine Environmental Research, 134: 28–36.
Orr J C, Fabry V J, Aumont O, Bopp L, Doney S C, Feely R A, Gnanadesikan A, Gruber N, Ishida A, Joos F, Key R M, Lindsay K, Maier-Reimer E, Matear R, Monfray P, Mouchet A, Najjar R G, Plattner G K, Rodgers K B, Sabine C L, Sarmiento J L, Schlitzer R, Slater R D, Totterdell I J, Weirig M F, Yamanaka Y, Yool A. 2005. Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms. Nature437(7059): 681–686.
Parker L M, Ross P M, O’Connor W A, Pörtner H O, Scanes E, Wright J M. 2013. Predicting the response of molluscs to the impact of ocean acidification. Biology, 2(2): 651–692.
Pierrot D, Lewis E, Wallace D W R. 2006. MS Excel Program Developed for CO2 System Calculations. ORNL/ CDIAC-105, Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory, U.S. Department of Energy, Oak Ridge, Tennessee.
Range P, Chícharo M A, Ben-Hamadou R, Piló D, Matias D, Joaquim S, Oliveira A P, Oliveira L. 2011. Calcification, growth and mortality of juvenile clams Ruditapes decussatus under increased p CO2 and reduced pH: variable responses to ocean acidification at local scales? Journal of Experimental Marine Biology and Ecology396(2): 177–184.
Range P, Piló D, Ben-Hamadou R, Chícharo M A, Matias D, Joaquim S, Oliveira A P, Chícharo L. 2012. Seawater acidification by CO2 in a coastal lagoon environment: effects on life history traits of juvenile mussels Mytilus galloprovincialis. Journal of Experimental Marine Biology and Ecology, 424-525: 89–98.
Raven J, Caldeira K, Elderfield H, Hoegh-Guldberg O, Liss P S, Riebesell U, Shepherd J, Turley C M, Watson A J. 2005. Ocean acidification Due to Increasing Atmospheric Carbon Dioxide. The Royal Society, London, UK.
Ries J B, Cohen A L, McCorkle D C. 2009. Marine calcifiers exhibit mixed responses to CO2 -induced ocean acidification. Geology, 37(12): 1131–1134.
Ries J B, Ghazaleh M N, Connolly B, Westfield I, Castillo K D. 2016. Impacts of seawater saturation state (Ω A = 0.4-4.6) and temperature (10, 25°) on the dissolution kinetics of whole-shell biogenic carbonates. Geochimica et Cosmochimica Acta, 192: 318–337.
Solomon S, Qin D, Manning M, Chen Z, Marquis M, Averyt K B, Tignor M, Miller H L. 2007. Climate Change 2007: the Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge.
Uddin M J, Yang H S, Park K J, Kang C K, Kang H S, Choi K S. 2012. Annual reproductive cycle and reproductive efforts of the Manila clam Ruditapes philippinarum in Incheon Bay off the west coast of Korea using a histology-ELISA combined assay. Aquaculture, 364-365: 25–32.
Waldbusser G G, Steenson R A, Green M A. 2011. Oyster shell dissolution rates in estuarine waters: effects of pH and shell legacy. Journal of Shellfish Research30(3): 659–669.
Widdicombe S, Dashfield S L, McNeill C L, Needham H R, Beesley A, McEvoy A, Øxnevad S, Clarke K R, Berge J A. 2009. effects of CO2 induced seawater acidification on infaunal diversity and sediment nutrient fluxes. Marine Ecology Progress Series, 379: 59–75.
Widdicombe S, Spicer J I. 2008. Predicting the impact of ocean acidification on benthic biodiversity: what can animal physiology tell us? Journal of Experimental Marine Biology and Ecology, 366(1-2): 187–197.
Xu X, Yang F, Zhao L Q, Yan X W. 2016. Seawater acidification affects the physiological energetics and spawning capacity of the Manila clam Ruditapes philippinarum during gonadal maturation. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology196: 20–29.
Yang F, Zhao L Q, Yan X W, Wang Y. 2013. Bioaccumulation of trace elements in Ruditapes philippinarum from China: public health risk assessment implications. International Journal of Environmental Research and Public Health10(4): 1 392–1 405.
Zhao L Q, Zhang Y, Liang J, Xu X, Wang H, Yang F, Yan X W. 2014. Environmental cadmium exposure impacts physiological responses in Manila clams. Biological Trace Element Research, 159(1-3): 241–253.
Zhao X G, Shi W, Han Y, Liu S X, Guo C, Fu W D, Chai X L, Liu G X. 2017. Ocean acidification adversely influences metabolism, extracellular pH and calcification of an economically important marine bivalve, Tegillarca granosa. Marine Environmental Research, 125: 82–89.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Supported by the earmarked fund for Modern Agro-industry Technology Research System (No. CARS-48)
Rights and permissions
About this article
Cite this article
Yuan, H., Xu, X., Yang, F. et al. Impact of seawater acidification on shell property of the Manila clam Ruditapes philippinarum grown within and without sediment. J. Ocean. Limnol. 38, 236–248 (2020). https://doi.org/10.1007/s00343-019-8281-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00343-019-8281-z