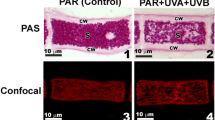
Abstract
We evaluated the effects of ultraviolet-B (UV-B) radiation and different light conditions on the repair of UV-B-induced damage in carpospores of Chondrus ocellatus Holm (Rhodophyta) in laboratory experiments. Carpospores were treated daily with different doses of UV-B radiation for 48 days, when vertical branches had formed in all treatments; after each daily treatment, the carpospores were subjected to photosynthetically active radiation (PAR), darkness, red light, or blue light during a 2-h repair stage. Carpospore diameters were measured every 4 days. We measured the growth and cellular contents of cyclobutane pyrimidine dimers (CPDs), chlorophyll a, phycoerythrin, and UV-B-absorbing mycosporine-like amino acids (MAAs) in carpospores on Day 48. Low doses of UV-B radiation (36 and 72 J/m2) accelerated the growth of C. ocellatus. However, as the amount of UV-B radiation increased, the growth rate decreased and morphological changes occurred. UV-B radiation significant damaged DNA and photosynthetic pigments and induced three kind of MAAs, palythine, asterina-330, and shinorine. PAR conditions were best for repairing UV-B-induced damage. Darkness promoted the activity of the DNA darkrepair mechanism. Red light enhanced phycoerythrin synthesis but inhibited light repair of DNA. Although blue light, increased the activity of DNA photolyase, greatly improving remediation efficiency, the growth and development of C. ocellatus carpospores were slower than in other light treatments.
Similar content being viewed by others
References
Aguilera J, Bischof K, Karsten U, Hanelt D, Wiencke D. 2002a. Seasonal variation in ecophysiological patterns in macroalgae from an Arctic fjord. II. Pigment accumulation and biochemical defence systems against high light stress. Mar. Biol., 140(6): 1 087–1 095.
Aguilera J, Dummermuth A, Karsten U, Schriek R, Wiencke C. 2002b. Enzymatic defences against photooxidative stress induced by ultraviolet radiation in Arctic marine macroalgae. Polar Biol., 25(6): 432–441.
Aguilera J, Jiménez C, Figueroa L F, Lebert M, Häder D P. 1999. Effect of ultraviolet radiation on thallus absorption and photosynthetic pigments in the red alga Porphyra umbilicalis. Journal of Photochemistry and Photobiology B: Biology, 48(1): 75–82.
Allen D J, McKee I F, Farage P K, Baker R N. 1997. Analysis of limitations to CO2 assimilation on exposure of leaves of two Brassica napus cultivars to UV-B. Plant Cell Envirol., 20(5): 633–640.
Altamirano M, Flores-Moya A, Figueroa F L. 2003. Effects of UV radiation and temperature on growth of germlings of three species of Fucus (Phaeophyta). Aquat. Bot., 75(1): 9–20.
An X L, Zhou Q X. 2006. Progress in study of bioremediation for aquaculture self-polluted water bodies. Techniques and Equipment for Environmental Pollution Control, 7(9): 1–6. ( in Chinese with English abstract)
Beer S, Eshel A. 1985. Determining phycoerythrin and phycocyanin concentrations in aqueous crude extracts of red algae. Aus. J. Mar. Freshw. Res., 36(6): 785–792.
Bischof K, Gómez I, Molis M, Hanelt D, Karsten U, Lüder U, Roleda M Y, Zacher K, Wiencke C. 2006. Ultraviolet radiation shapes seaweed communities. Rev. Environ. Sci. Biotechnol., 5(2–3): 141–166.
Bischof K, Hanelt D, Wiencke C. 1999. Acclimation of maximal quantum yield of photosynthesis in the brown alga Alaria esculenta under high light and UV radiation. Plant Biol., 1(4): 435–444.
Britt A B. 1995. Repair of DNA damage induced by ultraviolet radiation. Plant Physiol., 108(3): 891–896.
Britt A B. 1999. Molecular genetics of DNA repair in higher plants. Trends Plant Sci., 4(1): 20–25.
Buma A G J, van Hannen E J, Roza L, Veldhuis W J M, Gieskes W W C. 1995. Monitoring ultraviolet-B-induced DNA damage in individual diatom cells by immunofluorescent thymine dimer detection. J. Physiol., 31(2): 314–321.
Buma A G J, van Oijen T, van De Poll W, Veldhuis W J M, Gieskes W W C. 2000. The sensitivity of Emiliania huxleyi (Prymnesiophyceae) to ultraviolet-B radiation. J. Physiol., 36(2): 296–303.
Butler J H, Battle M, Bender M L, Montzka S A, Clarke A D, Saltzman E S, Sucher C M, Severinghaus J P, Elkins J W. 1999. A record of atmospheric halocarbons during the twentieth century from polar firn air. Nature, 399(6738): 749–755.
Cen Y P, Bornman J F. 1990. The response of bean plants to UV-B radiation under different irradiances of background visible light. J. Exp. Bot., 232(11): 1 489–1 495.
Cordi B, Donkin M E, Peloquin J, Price D N, Depledge M H. 2001. The influence of UV-B radiation on the reproductive cells of the intertidal macroalga, Enteromorpha intestinalis. Aquat. Toxicol., 56(1): 1–11.
Duffy J E, Hay M E. 2000. Strong impacts of grazing amphipods on the organization of a benthic community. Ecol. Monogr., 70(2): 237–263.
Dunlap W C, Shick J M. 1998. Review—Ultraviolet radiationabsorbing mycosporine-like amino acids in coral reef organisms: a biochemical and environmental perspective. J. Physiol., 34(3): 418–430.
Dunlap W C, Yamamoto Y. 1995. Small-molecule antioxidants in marine organisms: antioxidant activity of mycosporineglycine. Comp. Biochem. Physiol. B, 112(1): 105–114.
Eswaran K, Mairh O P, Rao P V S. 2002. Inhibition of pigments and phycocolloid in a marine red alga Gracilaria edulis by ultraviolet-B radiation. Biol. Plantarum, 45(1): 157–159.
Eswaran K, Rao P V S. 2001. Impact of ultraviolet-B radiation on a marine red alga Kappaphycus alvarezii (Solieriaceae, Rhodophyta). Indian J. Mar. Sci., 30(2): 105–107.
Freeman S E, Hacham H, Gange R W, Maytum D J, Sutherland J C, Sutherland B M. 1989. Wavelength dependence of pyrimidine dimer formation in DNA of human skin irradiated in situ with ultraviolet light. Proc. Natl. Acad. Sci., 86: 5 605–5 609.
Frohnmeyer H, Staiger D. 2003. Ultraviolet-B radiation-mediated responses in plants. Balancing damage and protection. Plant Physiol., 133(4): 1 420–1 428.
He J M, She X P, Meng Z N, Zhao W M. 2004. Reduction of Rubisco amount by UV-B radiation is related to increased H2O2 content in leaves of mung bean seedlings. J. Plant Physiol. Mol. Biol., 30(3): 291–296.
He J M, Wang R B, Meng Z N. 2006. Cyclobutyl pyrimidine dimer accumulation in relation to UV-B sensitivity in mung bean cultivars. Scientia Agricultur a Sinica, 39(7): 1 358–1 364. ( in Chinese with English abstract)
Hoyer K, Karsten U, Sawall T, Wiencke C. 2001. Photoprotective substances in Antarctic macroalgae and their variation with respect to depth distribution, different tissues and developmental stages. Mar. Ecol. Pro. Ser., 211: 117–129.
Hu D L. 2007. The current situation of ozonosphere hole. World Science, (4): 14. (in Chinese)
Karsten U, Sawall T, Wiencke C. 1998. A survey of the distribution of UV-absorbing substances in tropical macroalgae. Phycol. Res., 46(4): 271–279.
Karsten U, Wiencke C. 1999. Factors controlling the formation of UV-absorbing mycosporine-like amino acids in the marine red alga Palmaria palmata from Spitsbergen (Norway). J. Plant Physiol., 155(3): 407–415.
Karsten U. 2008. Defense strategies of algae and cyanobacteria against solar ultraviolet radiation. In: Amsler C D ed. Algal Chemical Ecology. 1st edn. Springer Berlin Heidelberg, Berlin, USA. p.273–296.
Li S S, Wang Y, Wang X J, Zhu Y B, Liu S H. 2000. DNA damage and repair in rice seedlings irradiated with UV-B. Acta Photonica Sinica, 29(7): 595–598. (in Chinese with English abstract)
Liu S, Zhang Q S, Wang Y, Ju Q, Tang X X. 2008. The response of the early developmental stages of Laminaria japonica to enhanced ultraviolet-B radiation. Sci. China Ser. C Life Sci., 51(12): 1 129–1 136.
Mayes C, Saunders G W, Tan I T, Druehl L D. 2004. DNA extraction methods for kelp ( Laminariales ) tissue. J. Physiol., 28(5): 712–716.
Mori T, Nakane M, Hattori T, Matsunaga T, Ihara M, Nikaido O. 1991. Simultaneous establishment of monoclonal antibodies specific for either cyclobutane pyrimidine dimer or (6-4) photoproduct from the same mouse immunized with ultraviolet-irradiated DNA. Photochem. Photobiol., 54(2): 225–232.
Munier M, Jubeau S, Wijaya A, Morançis M, Dumay J, Marchal L, Jaouen P, Fleurence J. 2014. Physicochemical factors affecting the stability of two pigments: R-phycoerythrin of Grateloupia turuturu and B-phycoerythrin of Porphyridium cruentum. Food Chem., 150: 400–407.
Pakker H, Beekman C A C, Breeman A M. 2000. Efficient photoreactivation of UVBR-induced DNA damage in the sublittoral macroalga Rhodymenia pseudopalmata (Rhodophyta). Eur. J. Physiol., 35(2): 109–114.
Pereira D T, Schmidt ÉC, Bouzon Z L, Ouriques L C. 2014. The effects of ultraviolet radiation-B response on the morphology, ultrastructure, and photosynthetic pigments of Laurencia catarinensis and Palisada flagellifera (Ceramiales, Rhodophyta): a comparative study. J. Appl. Phycol., 26(6): 2 443–2 452.
Polo L K, de Felix M R, Kreusch M, Pereira D T, Costa G B, Simioni C, Ouriques L C, Chow F, Ramlov F, Maraschin M, Bouzon Z L, Schmidt É C. 2014. Photoacclimation responses of the brown macroalga Sargassum cymosum to the combined influence of UV radiation and salinity: cytochemical and ultrastructural organization and photosynthetic performance. Photochem. Photobiol., 90(3): 560–573.
Pradhan M K, Joshi P N, Nair J S, Ramaswamy N K, Iyer R K, Biswal B, Biswal U C. 2006. UV-B exposure enhances senescence of wheat leaves: modulation by photosynthetically active radiation. Radiat. Environ. Biophys., 45(3): 221–229.
Rastogi P R, Incharoensakdi A. 2014. UV radiation-induced biosynthesis, stability and antioxidant activity of mycosporine-like amino acids (MAAs) in a unicellular cyanobacterium Gloeocapsa sp. CU2556. J. Photochem. Photobiol. B. Biol., 130(5): 287–292.
Renger G, Voss M, Gräber P, Schulze A. 1986. Effect of UV irradiation on differential partial reactions of the primary processes of photosynthesis. In: Worrest R C, Caldwell M M eds. Stratospheric ozone reduction, solar ultraviolet radiation and plant life. 1st edn. Springer, Berlin Heidelberg, Heidelberg, USA. p.171–184.
Ritchie R J. 2006. Consistent sets of spectrophotometric chlorophyll equations for acetone, methanol and ethanol solvents. Photosynth. Res., 89(1): 27–41.
Roleda M Y, van de Poll W H, Hanelt D, Wiencke C. 2004. PAR and UVBR effects on photosynthesis, viability, growth and DNA in different life stages of coexisting Gigartinales: implications for recruitment and zonation pattern. Mar. Ecol. Prog. Ser., 281: 37–50.
Scariot L Â, Rover T, Zitta C S, Horta P A, de Oliveira E C, Bouzon Z L. 2013. Effects of UV-B radiation on Gelidium floridanum (Rhodophyta, Gelidiales): germination of tetraspores and early sporeling development. J. Appl. Physiol., 25(2): 537–544.
Schmidt É C, dos Santos R, Horta P A, Maraschin M, Bouzon Z L. 2010a. Effects of UVB radiation on the agarophyte Gracilaria domingensis (Rhodophyta, Gracilariales): changes in cell organization, growth and photosynthetic performance. Micron, 41(8): 919–930.
Schmidt É C, dos Santos R W, de Faveri C, Horta P A, de Paula Martins R, Latini A, Ramlov F, Maraschin M, Bouzon Z L. 2012a. Response of the agarophyte Gelidium floridanum after in vitro exposure to ultraviolet radiation B: changes in ultrastructure, pigments, and antioxidant systems. J. Appl. Phycol., 24(6): 1 341–1 352.
Schmidt É C, Maraschin M, Bouzon Z L. 2010b. Effects of UVB radiation on the carragenophyte Kappaphycus alvarezii (Rhodophyta, Gigartinales): changes in ultrastructure, growth, and photosynthetic pigments. Hydrobiologia, 649(1): 171–182.
Schmidt É C, Nunes B G, Maraschin M, Bouzon Z L. 2010c. Effect of ultraviolet-B radiation on growth, photosynthetic pigments, and cell biology of Kappaphycus alvarezii (Rhodophyta, Gigartinales) macroalgae brown strain. Photosynthetica, 48(2): 161–172.
Schmidt É C, Pereirab B, dos Santosc R W, Gouveiac C, Costac G B, Fariac G S M, Schernerd F, Hortad P A, de Paula Martinse R, Latini A, Ramlovf F, Maraschinf M, Bouzon Z L. 2012b. Responses of the macroalgae Hypnea musciformis after in vitro exposure to UV-B. Aquat. Bot., 100: 8–17.
Schmidt É C, Scariot L A, Rover T, Bouzon Z L. 2009. Changes in ultrastructure and histochemistry of two red macroalgae strains of Kappaphycus alvarezii (Rhodophyta, Gigartinales), as a consequence of ultraviolet B radiation exposure. Micron, 40(8): 860–869.
Shao H B. 2001. The regulation and control of flowering time and photoreceptors in higher plants. II. the molecular mechanism of photoreceptor controlling flowering courseand circadian clocks. Life Science Research, 5(3): 154–160. (in Chinese with English abstract)
Shick J M, Dunlap W C. 2002. Mycosporine-like amino acids and related gadusols: Biosynthesis, accumulation, and UV-protective functions in aquatic organisms. Annu. Rev. Physiol., 64: 223–262.
Simioni C, Schmidt É C, de Felix M R, Polo L K, Rover T, Kreusch M, Pereira D T, Chow F, Ramlov F, Maraschin M, Bouzon Z L. 2014. Effects of ultraviolet radiation (UVA+UVB) on young Gametophytes of Gelidium floridanum: growth rate, photosynthetic pigments, carotenoids, photosynthetic performance, and ultrastructure. Photochem. Photobiol., 90(5): 1 050–1 060, http://dx.doi.org/10.111110.1111/php.12296.
Sinha R P, Ambasht N K, Sinha J P, Klisch M, Häer D P. 2003. UV-B-induced synthesis of mycosporine-like amino acids in three strains of Nodularia (cyanobacteria). J. Photochem. Photobiol., 71(1–3): 51–58.
Sinha R P, Hader P D. 1998. Effects of ultravjolet-B radiation in three rice field cyanobacteria. J. Plant Physiol., 153(5–6): 763–769.
Smith S V. 1981. Marine macrophytes as a global carbon sink. Science, 211(4484): 838–840.
Sousa W P, Connell J H. 1992. Grazing and succession in marine algae. In: John D M, Hawkins S J, Price J H eds. Plant-animal Interactions in the Marine Benthos. 1st ed. Clarendon Press, Oxford, USA. p.425–441.
Starr R C, Zeikus J A. 1993. UTEX- the culture collection of algae at the university of Texas at Austin 1993 list of cultures. J. Physiol., 29(S2): 1–106.
Strid Å, Chow W S, Anderson J M. 1994. UV-B damage and protection at the molecular level in plants. Photosynth. Res., 39(3): 475–489.
Sui Z H, Zhang X C. 1998. Review on phycoerythrin research. Marine Sciences, (4): 24–27. (in Chinese with English abstract)
Tang G M, Zhong Y, Zeng X Q. 2008. Study on biodiversity of macrobenthic fauna in the rocky intertidal zone of Qingdao. South China Fisheries Science, 4(5): 8–15. (in Chinese with English abstract)
Teramura A H. 1980. Effects of ultraviolet-B irradiances on soybean. Physiol. Plantarum, 48(2): 333–339.
Teramura A H. 1983. Effects of ultraviolet-B radiation on the growth and yield of crop plants. Physiol. Plantarum, 58(3): 415–427.
Thompson C L, Sancar A. 2002. Photolyase/cryptochrome blue-light photoreceptors use photon energy to repair DNA and reset the circadian clock. Oncogene, 21(58): 9 043–9 056.
Whitehead K, Hedges J I. 2002. Analysis of mycosporine-like amino acids in plankton by liquid chromatography electrospray ionization mass spectrometry. Mar. Chem., 80(1): 27–72.
Wiencke C, Gómez I, Pakker H, Flores-Moya A, Altamirano M, Hanelt D, Bischof K, Figueroa F L. 2000. Impact of UV-radiation on viability, photosynthetic characteristics and DNA of brown algal zoospores: implications for depth zonation. Mar. Ecol. Prog. Ser., 197: 217–229.
Wiencke C, Roleda M Y, Gruber A, Clayton M N, Bischof K. 2006. Susceptibility of zoospores to UV radiation determines upper depth distribution limit of Arctic kelps: evidence through field experiments. J. Ecol., 94(2): 455–463.
Xu D, Tang X X, Zhang P Y. 2003. The physiological effect of UV-B radiation on microalgae two species of marine microalgae. Journal of Ocean University of Qingdao, 33(2): 240–244. ( in Chinese with English abstract)
Xu J T, Gao K S. 2009. Growth, pigments, UV-absorbing compounds and agar yield of the economic red seaweed Gracilaria lemaneiformis ( Rhodophyta ) grown at different depths in the coastal waters of the South China Sea. J. Appl. Phycol., 20(5): 681–686.
Yokthongwattana W, Chrost B, Behrman S, Casper-Lindley C, Melis A. 2001. Photosystem II damage and repair cycle in the green alga Dunaliella salina: involvement of a chloroplast-localized HSP70. Plant Cell Physiol., 42(12): 1 389–1 397.
Zenoff V F, Siñeriz F, Farías M E. 2006. Diverse responses to UV-B radiation and repair mechanisms of bacteria isolated from high-altitude aquatic environments. Appl. Environ. Microbiol., 72(12): 7 857–7 863.
Zhong C, Chen Z Y, Wang Y, Liu Y Z. 2009. Molecular level study of the effects of UV-B radiation on plant: a review. Chin. J. Ecol., 28(1): 129–137. (in Chinese with English abstract)
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by the Program for New Century Excellent Talents in University (No. NCET-05-0597) and the National Natural Science Foundation of China (No. 30270258)
Rights and permissions
About this article
Cite this article
Ju, Q., Xiao, H., Wang, Y. et al. Effects of different light conditions on repair of UV-B-induced damage in carpospores of Chondrus ocellatus Holm. Chin. J. Ocean. Limnol. 33, 664–678 (2015). https://doi.org/10.1007/s00343-015-4261-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00343-015-4261-0