Abstract
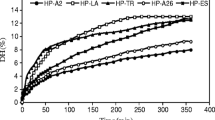
Preparation of Fe2+ chelate of fish protein hydrolysate (Fe-FPH) obtained from low value fish proteins was introduced and its bioactivity was studied by compound enzymolysis. The optimum conditions for hydrolysate chelating Fe2+ are DH (degree of hydrolysis) at 5%, pH 7.0, 20°C and 15 min chelating time for FM (material not being defatted). Four types of Fe-FPH including CA (deposit after chelating), CB (deposit in 50% of absolute ethanol solution), CC (suspended deposit in 80% of absolute ethanol solution), and CD (bottom deposit in 80% of absolute ethanol solution) were fractionated with absolute ethanol from FM. Structural analysis through infra-red spectrum revealed that Fe2+ was combined strongly with amino-group and carboxyl-group in each chelate and each Fe2+ could form two five-member ring structures. All of the four chelates were shown more significant antioxidative activity and can be used as natural hydrophobic and hydrophilic antioxidant. Among all the chelates, the CB possesses the most effective antioxidative activity at 92% as high as that of a-tocopherol. Among all Fe-FPHs, only CD showed the most effective antibacterial activity against Escherichia coli, Staphylococcus aureus, Salmonella typhi, and Bacillus subtilis and can be used as natural antibacterial. It provides a more effective way for utilization of low value fish proteins and key information of Fe-FPH as additive in food industry.
Similar content being viewed by others
References
Baek, H. H. and K. R. Cadwallader, 1995. Enzymic hydrolysis of crayfish processing by-products. J. Food Sci. 60: 929–934.
Deng, S. G., Z. Y. Peng, F. Chen, P. Yang and T. Wang, 2004a. Amino acids composition and anti-anaemia action of hydrolyzed offal protein from Harengula zunasi Bleeker. Food Chemistry 87(1): 97–102.
Deng, S. G, Z. Y. Peng, F. Chen, Z. J. Chang and P. Yang, 2004b. Hydrolysis dynamics model of protein from Harengula zunasi by compound enzymes. J. Zhanjiang Ocean University 24(4): 28–32. (in Chinese)
Freeman, H. C. and P. L. Hoogland, 1956. Processing of cod and haddock viscera: 1. Laboratory experiments. J. Fish Res. Board Can. 13: 86–877.
Hale, M. B., 1969. Relative activities of commercial-available enzymes in the hydrolysis of fish protein. Food Technol. 23: 107–110.
Hale, M. B., 1974. Using enzymes to make fish protein concentrates. Mar Fish Rev. 36: 15–19.
He, N., C. L. Wang, X. B. Gu, F. P. Lu and L. X. Du, 1999. Study on the optimization of the activity of lactobacillin with well test. J. Tianjin Institute of Light Industry 2: 17–20.
Hevia, P., J. Whitaker and H. S. Olott, 1976. Solubilization of a fish protein concentrate with proteolytic enzymes. J. Agric. Food Chem. 24: 383–390.
Hordur, G. K. and A. R. Barbara, 2000. Kinetics of the hydrolysis of Atlantic salmon (Salmo salar) muscle proteins by alkaline proteases and a visceral serine protease mixture. Process Biochemistry 36: 131–139.
Jeon, Y. J., H. G. Byun and S. K. Kim, 1999. Improvement of functional properties of cod frame protein hydrolysates using ultrafiltration membranes. Process Biochemistry 35: 471–478.
Kim, S. K., Y. J. Jeon, H. G. Byun, Y. T. Kim and C. K. Lee, 1997. Enzymatic recovery of cod frame protein by crude proteinase derived from tuna pyloric caeca. Fish Sci. 63: 421–427.
Kristinsson, H. G. and B. A. Rasco, 2000a. Biochemical and functional properties of Atlantic salmon (Salmo salar) muscle proteins hydrolyzed with various alkaline proteases. J. Agric. Food Chem. 48: 657–666.
Kristinsson, H. G. and B. A. Rasco, 2000b. Kinetics of the hydrolysis of Atlantic salmon (Salmo salar) muscle proteins by alkaline proteases and a visceral serine protease mixture. Proc. Biochem. 36: 131–139.
Kristinsson, H. G. and B. A. Rasco, 2000c. Fish protein hydrolysates: production, biochemical, and functional properties. CRC Crit Rev Food Sci. Nutr. 40: 43–81.
Liaset, B., E. Lied and M. Espe, 2000. Enzymatic hydrolysis of by-products from the fish-filleting industry; chemical characterisation and nutritional evaluation. J. Sci. Food Agric. 80: 581–589.
McBride J. R., D. R. Idler and R. A. MacLeod, 1961. The liquefaction of British Columbia herring by ensilage, proteolytic enzymes and acid hydrolysis. J. Fish. Res. Board Can. 18: 93–112.
Mohr, V., 1977. Fish protein concentrate production by enzymic hydrolysis. In: Adler-Nissen J, (eds). Biochemical Aspects of New Protein Food. New York: Pergamon. p. 53–62.
Ohkawa, H., N. Ohishi and K. Yagi, 1979. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 95: 351–358.
Osawa, T and M. Namiki, 1981. A novel type of antioxidant isolated from leaf wax of eucalyptus leaves. Agric. Biol. Chem. 45: 735–773.
Quaglia, G. B. and E. Orban, 1987a. Influence of the degree of hydrolysis on the solubility of the protein hydrolsyates from sardine (Sardina pilchardus). J. Sci. Food Agric. 38: 271–276.
Quaglia, G. B. and E. Orban, 1987b. Enzymic solubilisation of proteins of sardine (Sardina pilchardus) by commercial proteases. J. Sci. Food Agric. 38: 263–269.
Sen, D. P., N. V. Sripathy, N. L. Lahiry et al, 1962. Fish hydrolysates I: Rate of hydrolysis of fish flesh with papain. Food Technol. 16(5): 138–140.
Tarr, H. L. A., 1948. Possibilities in developing fisheries by-products. Food Techol. 2: 268–277.
Uchida, K. and S. Kawakishi, 1992. Sequence-dependent reactivity of histidine-containing peptides with copper(II): ascorbate. J. Agric. Food Chem. 40: 13–16.
Zhao, H. G., and M. R. Huang, 1987. Aquatic Product Inspection. 1st edn. Tianjin Science and Technology Press, Tianjin. p. 165–166.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by National Natural Science Foundation of China (No.30371123) and Science and Technology Department of Zhejiang Province (No. 2007C12013).
Rights and permissions
About this article
Cite this article
Deng, S., Huo, J. & Xie, C. Preparation by enzymolysis and bioactivity of iron complex of fish protein hydrolysate (Fe-FPH) from low value fish. Chin. J. Ocean. Limnol. 26, 300–306 (2008). https://doi.org/10.1007/s00343-008-0300-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00343-008-0300-4