Abstract
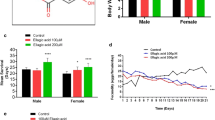
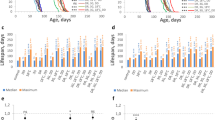
The effects of degraded porphyran (P1) and natural porphyran (P) on the lifespan and vitality of Drosophila melanogaster are studied. The porphyrans, added daily to the food medium at 0.2% and 1% concentrations, can significantly increase the lifespan in average of 55.79 and 58.23 d in 0.2% P1 diet females and 1% P1 diet_males, extending by 12.29% and 8.60% over the corresponding controls, respectively. The effects of porphyrans on D. melanogaster in heat-stress condition were also examined, and found a remarkable increase in survival time. The results which are consistently associated with the use of porphyrans are related to their free radical scavenger action. Considerable increase in vitality demonstrated that vitalities of middle-aged fly (assessed by measuring their mating capacity) was observed after porphyrans addition. Therefore, porphyrans are effective in reducing the rate of aging, and P1 in low molecular weight is better than natural P.
Similar content being viewed by others
References
Arking R., 1998. Biology of Aging: Observations and Principles, 2nd edition. Sinauer Associates, Sunderland, MA.
Bonilla, E., S. Medina-Leendertz and S. Diaz, 2002. Extension of life span and stress resistance of Drosophila melanogaster by long-term supplementation with melatonin. Exp. Gerontol. 37: 629–638.
Brack, C., E. Bechter-Thuring and M. Labuhn, 1997. N-acetylcysteine. slows down aging and increase the life span of Drosophila. melanogaster. Cell. Mol. Life Sci. 53: 960–966.
Davies, S., R. Kattel, B. Bhatia, A. Petherwick and T. Chapman, 2005. The effect of diet, sex and mating status on longevity in Mediterranean fruit flies (Ceratitis capitata), Diptera: Tephritidae. Exp. Gerontol. 40: 784–792.
Dudas, S. P. and R. Arking, 1995. A coordinate upregulation of antioxidant gene activities is associated with the delayed onset of senescence in a long-lived strain of Drosophila. J. Gerontol. A. Biol. Sci. Med. Sci. 50: B117–B127.
Fleming, J. E., I. Reveillaud and A. Niedzwieeki, 1992. Role of oxidative stress in Drosophila aging. Mut. Res. 275: 267–79.
Fridovich, I., 1975. Superoxide dismutases. Ann. Rev. Biochem. 44: 147–159.
Harman, D., 1956, Aging: A Theory Based on Free Radical and Radiation Chemistry. J. Gerontol. 11: 298–300.
Izmaylov, D. M. and L. K. Obukhova, 1999. Geroprotector effectiveness of melatonin: investigation of lifespan of Drosophila melanogaster. Mech. Ageing Dev. 106: 233–240.
Miquel, J., P. R., Lundgren, K. G. Bensch and H. Atlan. 1976. Effects of temperature on the life-span, vitality and fine structure of Drosophila melanogaster. Mech. Ageing Dev. 5: 347–370.
Miyatake, T., T. Chapman and L. Partridge, 1999. Mating induced inhibition of remating in female Mediterranean fruit fly Ceratitis capitata. J. Insect Physiol. 45: 1021–1028.
Muller, H. G., J. L. Wang, W. B. Capra, P. Liedo and J. R. Carey, 1997. Early mortality surge in protein deprived females causes reversal of sex differential of life expectancy in Mediterranean fruit flies. Proc. Natl. Acad. Sci. USA. 94: 2 762–2 765.
Parkes, T. L., A. J. Elia, D. Dickinson, A. J. Hilliker, J. P. Phillips and G. L. Boulianne, 1998. Extension of Drosophila lifespan by overexpression of human SOD1 in motorneurons. Nat. Genet. 19: 171–174.
Rose, M. R., L. N. S. U. Vu, J. L. Park and J. L. Graves, Jr 1992. Selection on stress resistance increases longevity in Drosophila melanogaster. Exp. Gerontol. 27: 241–250.
Ruddle, D. L., L. S. Yengoyan, J. Miquel, R. Marcuson and J. E. Fleming, 1988. Propyl gallate delays senescence in Drosophila melanogaster. Age 11: 54–58.
Simon, A. F., C. Shih, A. Mack and S. Benzer, 2003. Steroid Control of Longevity in Drosophila melanogaster. Science 299: 1 407–1 410.
Service, P. M., E. W. Hutchinson, M. D. Mackinley and M. R. Rose, 1985. Resistance to environmental stress in Drosophila melanogaster selected for postponed senescence. Physiol. Zool. 58: 380–389.
Sun, J. and J. Tower 1999. FLP Recombinase-mediated induction of Cu/Zn-superoxide dismutase transgene expression can extend the lifespan of adult Drosophila melanogaster flies. Mol. Cell. Bio. 19: 216–228.
Tan, D. X., L. D. Chen, B. Poeggeler, L. C. Manchester and R. J. Reiter, 1993. Melatonin: a potent, endogenous hydrexyl radical scavenger. Endocr. J. 1: 57–60.
Tatar, M., A. Bartke and A. Antebi, 2003. The endocrine regulation of aging by insulin-like signals. Science 299: 1 346–1 351.
Yashizawa, Y., A. Enomoto, H. Todoh, A. Ametani and S. Kaminogawa, 1993. Activation of murine macrophages by polysaccharide fractions from marine alga (Porphyra yezoensis). Biosci. Biotech. Biochem. 57: 1 862–1 866.
Yashizawa, Y., A. Ametani, J. Tsunehiro, K. Numura, M. Itoh, F. Fukui and S. Kaminogawa, 1995. Macrophage stimulation activity of the polysaccharide fraction from a marine alga (Porphyra yezoensis): structure-function relationships and improved solubility. Biosci. Biotech. Biochem. 59: 1 933–1 937.
Zhang, Q., P. Yu, Z. Li, H. Zhang, Z. Xu and P. Li, 2003a. Antioxidant activities of sulfated polysaccharide fractions from Porphyra haitanesis. J. Appl. Phycol. 15: 305–310.
Zhang, Q., N. Li, G. Zhou, X. Lu, Z. Li and Z. Xu, 2003b. In vivo antioxidant activity of polysaccharide fraction from Porphyra haitanesis (Rhodephyta) in aging mice. Pharmacol. Res. 48: 151–155.
Zhang, Q., N. Li, X. Liu, Z. Zhao, Z. Li and Z. Xu, 2004. The structure of a sulfated galactan from Porphyra haitanensis and its in vivo antioxidant activity. Carbohydr. Res. 339: 105–111.
Zhao, T., Q. Zhang, H. Qi, H. Zhang, X. Niu, Z. Xu and Z. Li, 2006. Degradation of porphyran from Porphyra haitanensis and the antioxidant activities of the degraded porphyrans with different molecular weights. Int. J. Biol. Macromol. 38: 45–50
Zhou, H. and Q. Chen, 1989. The cytoprotection of polysaccharide from Porphyra yezoenisis Ueda. J. Chin. Pharma. Uni. 20: 340–343.
Zhou, H. and Q. Chen, 1990. Anticoagulant and antihyperlipedemic effects of polysaccharide from Porphyra yezoensis. J. China. Pharm. Univ. 21: 358–360.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by the Innovative Key Project of the Chinese Academy of Sciences (KZCX-YW-209).
Rights and permissions
About this article
Cite this article
Zhao, T., Zhang, Q., Qi, H. et al. Positive effect of porphyrans on the lifespan and vitality of Drosophila melanogaster . Chin. J. Ocean. Limnol. 25, 373–377 (2007). https://doi.org/10.1007/s00343-007-0373-5
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s00343-007-0373-5