Abstract
An optical sensor was developed for simultaneous detection of sulfur dioxide (SO2) and sulfur trioxide (SO3) at elevated temperatures using a quantum cascade laser (QCL) spectrometer. The SO2/SO3 concentrations and the SO2 conversions were measured during the heterogeneous catalytic reaction with S-101 intermediate-temperature vanadium catalysts at pressures of 3–20 kPa and temperatures of 550–1000 K. The SO2 spectra in the ν3 band and the SO3 spectra in the ν3 + ν4 band near 7.16 µm at elevated temperatures were well resolved in the strongly overlapping spectra by measuring the SO2 and SO3 high-temperature individual absorption spectra that were extended from room-temperature spectra in the HITRAN database. A SO2/SO3 separation unit was designed to extract and measure the remaining SO2 after conversion at room temperature, with the results comparing well with the optically measured concentrations in the high-temperature catalytic reactions. The system was then used to investigate the pressure and temperature dependencies of the heterogeneous conversions of SO2. SO3 was first detected at temperatures higher than 590 K with the SO2 conversion increasing to 92% at 797 K and then decreasing to less than 3% at 1014 K. The measured conversions show a logarithmic growth with increasing pressures at each temperature. The difference between the measured heterogeneous conversion and the theoretical conversion calculated using a thermodynamic model was also analyzed.


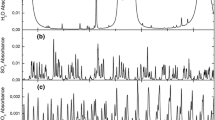
taken from the HITRAN/HITEMP database











Similar content being viewed by others
References
C. Zheng, Y. Wang, Y. Liu, Z. Yang, R. Qu, D. Ye, C. Liang, S. Liu, X. Gao, Formation, transformation, measurement, and control of SO3 in coal-fired power plants. Fuel 241(1), 327–346 (2019). https://doi.org/10.1016/j.fuel.2018.12.039
D. Bongartz, A.F. Ghoniem, Chemical kinetics mechanism for oxy-fuel combustion of mixtures of hydrogen sulfide and methane. Combustion Flame 162(3), 544–553 (2015). https://doi.org/10.1016/j.combustflame.2014.08.019
X. Zheng, E.M. Fisher, F.C. Gouldin, J.W. Bozzelli, Pyrolysis and oxidation of ethyl methyl sulfide in a flow reactor. Combustion Flame 158(6), 1049–1058 (2011). https://doi.org/10.1016/j.combustflame.2010.10.018
D. Bongartz, A.F. Ghoniem, Impact of sour gas composition on ignition delay and burning velocity in air and oxy-fuel combustion. Combustion Flame 162(7), 2749–2757 (2015). https://doi.org/10.1016/j.combustflame.2015.04.014
J. Mu, D.D. Perlmutter, Thermal decomposition of inorganic sulfates and their hydrates. Ind. Eng. Chem. Process Design Dev. 20(4), 640–646 (1981). https://doi.org/10.1021/i200015a010
J. Paulik, F. Paulik, M. Arnold, Simultaneous TG, DTG, DTA and EGA technique for the determination of carbonate, sulphate, pyrite and organic material in minerals, soil and rocks-III Operation of the thermo-gas-titrimetric equipment and the examination procedure in special cases. J Thermal Anal. 29(2), 345–351 (1984). https://doi.org/10.1007/BF02720070
L.P. Belo, L.K. Elliott, R.J. Stanger, R. Spörl, K.V. Shah, J. Maier, T.F. Wall, High-temperature conversion of SO2 to SO3: Homogeneous experiments and catalytic effect of fly ash from air and oxy-fuel firing. Energy Fuels 28(11), 7243–7251 (2014). https://doi.org/10.1021/ef5020346
D. Fleig, M.U. Alzueta, F. Normann, M. Abián, K. Andersson, F. Johnsson, Measurement and modeling of sulfur trioxide formation in a flow reactor under post-flame conditions. Combustion Flame 160(6), 1142–1151 (2013). https://doi.org/10.1016/j.combustflame.2013.02.002
L.P. Belo, R. Spörl, K.V. Shah, L.K. Elliott, R.J. Stanger, J. Maier, T.F. Wall, Sulfur capture by fly ash in air and oxy-fuel pulverized fuel combustion. Energy Fuels 28(8), 5472–5479 (2014). https://doi.org/10.1021/ef500855w
R.K. Srivastava, C.A. Miller, C. Erickson, R. Jambhekar, Emissions of sulfur trioxide from coal-fired power plants. J. Air. Waste Manag. Assoc. 54(6), 750–762 (2004). https://doi.org/10.1080/10473289.2004.10470943
R. Spörl, J. Walker, L. Belo et al., SO3 emissions and removal by ash in coal-fired oxy-fuel combustion. Energy Fuels 28(8), 5296–5306 (2014). https://doi.org/10.1021/ef500806p
A.W. Hodgson, P. Jacquinot, P.C. Hauser, Electrochemical sensor for the detection of SO2 in the low-ppb range. Anal. Chem. 71(14), 2831–2837 (1999). https://doi.org/10.1021/ac9812429
D. Weidmann, A. Hamdouni, D. Courtois, CH4/air/SO2 premixed flame spectroscopy with a 7.5-µm diode laser. Appl. Phys. B 73(1), 85–91 (2001). https://doi.org/10.1007/s003400100613
L. Joly, V. Zéninari, B. Parvitte, D. Weidmann, D. Courtois, Y. Bonetti, T. Aellen, M. Beck, J. Faist, D. Hofstetter, Spectroscopic study of the ν1 band of SO2 using a continuous-wave DFB QCL at 9.1 µm. Appl. Phys. B 77(6–7), 703–706 (2003). https://doi.org/10.1007/s00340-003-1310-8
B. Grouiez, B. Parvitte, L. Joly, D. Courtois, V. Zeninari, Comparison of a quantum cascade laser used in both cw and pulsed modes. Application to the study of SO2 lines around 9 µm. Appl. Phys. B 90(2), 177–186 (2008). https://doi.org/10.1007/s00340-007-2857-6
L. Richard, I. Ventrillard, G. Chau, K. Jaulin, E. Kerstel, D. Romanini, Optical-feedback cavity-enhanced absorption spectroscopy with an interband cascade laser: application to SO2 trace analysis. Appl. Phys. B 122(9), 1–7 (2016). https://doi.org/10.1007/s00340-016-6502-0
F. Xu, Y.G. Zhang, G. Somesfalean, Z.G. Zhang, H.S. Wang, S.H. Wu, Temperature-corrected spectroscopic evaluation method for gas concentration monitoring. Appl. Phys. B 86(2), 361–364 (2007). https://doi.org/10.1007/s00340-006-2489-2
H.S. Wang, Y.G. Zhang, S.H. Wu, X.T. Lou, Z.G. Zhang, Y.K. Qin, Using broadband absorption spectroscopy to measure concentration of sulfur dioxide. Appl. Phys. B 100(3), 637–641 (2010). https://doi.org/10.1007/s00340-010-4151-2
J. Henningsen, Measurement of free SO2 in wine with 74-µm difference frequency spectrometer. Appl. Phys. B 76(4), 451–456 (2003). https://doi.org/10.1007/s00340-003-1141-7
J. Henningsen, J. Hald, Quantitative analysis of dilute mixtures of SO2 in N2 at 7.4 µm by difference frequency spectroscopy. Appl. Phys. B 76(4), 441–449 (2003). https://doi.org/10.1007/s00340-003-1140-8
L. Wang, Y. Zhang, X. Zhou, F. Qin, Z. Zhang, Optical sulfur dioxide sensor based on broadband absorption spectroscopy in the wavelength range of 198–222 nm. Sens. Actuators, B 241, 146–150 (2017). https://doi.org/10.1016/j.snb.2016.10.055
Y. Matsumi, H. Shigemori, K. Takahashi, Laser-induced fluorescence instrument for measuring atmospheric SO2. Atmosp. Environ. 39(17), 3177–3185 (2005). https://doi.org/10.1016/j.atmosenv.2005.02.023
X.T. Lou, G. Somesfalean, Z.G. Zhang, S. Svanberg, Sulfur dioxide measurements using an ultraviolet light-emitting diode in combination with gas correlation techniques. Appl. Phys. B 94(4), 699–704 (2009). https://doi.org/10.1007/s00340-009-3399-x
Y. Cao, H. Zhou, W. Jiang et al., Studies of the fate of sulfur trioxide in coal-fired utility boilers based on modified selected condensation methods. Environ. Sci. Technol. 44(9), 3429–3434 (2010). https://doi.org/10.1021/es903661b
R.F. Maddalone, S.F. Newton, R.G. Rhudy, R.M. Statnick, Laboratory and field evaluation of the controlled condensation system for SO3 measurements in flue gas streams. J. Air Pollut. Control Assoc. 29(6), 626–631 (1979). https://doi.org/10.1080/00022470.1979.10470834
D. Fleig, E. Vainio, K. Andersson, A. Brink, F. Johnsson, M. Hupa, Evaluation of SO3 measurement techniques in air and oxy-fuel combustion. Energy Fuels 26(9), 5537–5549 (2012). https://doi.org/10.1021/ef301127x
C. Zheng, X. Li, Z. Yang, Y. Zhang, W. Wu, X. Wu, X. Wu, X. Gao, Development and experimental evaluation of a continuous monitor for SO3 measurement. Energy Fuels 31(9), 9684–9692 (2017). https://doi.org/10.1021/acs.energyfuels.7b01181
D. D. Stuart (2010) Acid dewpoint temperature measurement and its use in estimating sulfur trioxide concentration, ISA automation week 2010: Technology and Solutions Event 339–350.
E. Vainio, D. Fleig, A. Brink, K. Andersson, F. Johnsson, M. Hupa, Experimental evaluation and field application of a salt method for SO3 measurement in flue gases. Energy Fuels 27(5), 2767–2775 (2013). https://doi.org/10.1021/ef400271t
V. Bondybey, J. English, Infrared spectra of SO3 polymers and complexes in rare gas matrices. J. Mol. Spectrosc. 109, 221–228 (1985). https://doi.org/10.1016/0022-2852(85)90308-X
S.W. Sharpe, T.A. Blake, R.L. Sams, A. Maki, T. Masiello, J. Barber, N. Vulpanovici, J.W. Nibler, A. Weber, The ν3 and 2ν3 bands of 32S16O3, 32S18O3, 34S16O3, and 34S18O3. J. Mol. Spectrosc. 222(2), 142–152 (2003). https://doi.org/10.1016/j.jms.2003.08.001
F. Tetsuo, H. Ninomiya, SO3 concentration measurement using ultraviolet absorption spectroscopy and thermal conversion. IEEJ Trans. Fundamentals Materials 126, 977–982 (2006). https://doi.org/10.1541/IEEJFMS.126.977
J. Mellqvist, H. Axelsson, A. Rosén, Doas for flue gas monitoring - III In-situ monitoring of sulfur dioxide, nitrogen monoxide and ammonia. J. Quantitative Spectrosc. Radiative Trans. 56(2), 225–240 (1996). https://doi.org/10.1016/0022-4073(96)00044-1
D.M. Sonnenfroh, M.G. Allen, W.T. Rawlins, C.F. Gmachl, F. Capasso, A.L. Hutchinson, D.L. Sivco, A.Y. Cho, Pollutant emission monitoring using QC laser-based mid-IR sensors. Water Ground Air Pollut. Monit. Remediation 4199, 86 (2001). https://doi.org/10.1117/12.417364
O.G. Buzykin, S.V. Ivanov, A.A. Ionin, A.A. Kotkov, A.Y. Kozlov, Spectroscopic detection of sulfur oxides in the aircraft wake. J. Russian Laser Res 26(5), 402–426 (2005). https://doi.org/10.1007/s10946-005-0043-z
J. M. Hensley, W. T. Rawlins, D. B. Oakes, D. M. Sonnenfroh, M. G. Allen, A quantum cascade laser sensor for SO2 and SO3, 2005 Conference on Lasers and Electro-Optics, CLEO 2 (31) (2005) 1073–1075. doi:https://doi.org/10.1364/AO.44.006635
T. Hieta, M. Merimaa, Simultaneous detection of SO2, SO3 and H2O using QCL spectrometer for combustion applications. Appl. Phys. B 117(3), 847–854 (2014). https://doi.org/10.1007/s00340-014-5896-9
T.A. Berkoff, J.C. Wormhoudt, R.C. Miake-Lye, Measurement of SO2 and SO3 using a tunable diode laser system[C]//environmental monitoring and remediation technologies. Int. Soc. Opt. Photonics 3534, 686–693 (1999). https://doi.org/10.1117/12.339057
W.T. Rawlins, J.M. Hensley, D.M. Sonnenfroh et al., Quantum cascade laser sensor for SO2 and SO3 for application to combustor exhaust streams. Appl. Opt. 44(31), 6635–6643 (2005). https://doi.org/10.1364/AO.44.006635
A. Tokura, O. Tadanaga, T. Nishimiya, K. Muta, N. Kamiyama, M. Yonemura, S. Fujii, Y. Tsumura, M. Abe, H. Takenouchi, K. Kenmotsu, Y. Sakai, Investigation of SO3 absorption line for in situ gas detection inside combustion plants using a 4-µm band laser source. Appl. Opt. 55(25), 6887 (2016). https://doi.org/10.1364/ao.55.006887
I. E. Gordon, L. S. Rothman, C. Hill, R. V. Kochanov, Y. Tan, P. F. Bernath, M. Birk, V. Boudon, A. Campargue, K. V. Chance, B. J. Drouin, J. M. Flaud, R. R. Gamache, J. T. Hodges, D. Jacquemart, V. I. Perevalov, A. Perrin, K. P. Shine, M. A. Smith, J. Tennyson, G. C. Toon, H. Tran, V. G. Tyuterev, A. Barbe, A. G. Császár, V. M. Devi, T. Furtenbacher, J. J. Harrison, J. M. Hartmann, A. Jolly, T. J. Johnson, T. Karman, I. Kleiner, A. A. Kyuberis, J. Loos, O. M. Lyulin, S. T. Massie, S. N. Mikhailenko, N. Moazzen-Ahmadi, H. S. Müller, O. V. Naumenko, A. V. Nikitin, O. L. Polyansky, M. Rey, M. Rotger, S. W. Sharpe, K. Sung, E. Starikova, S. A. Tashkun, J. V. Auwera, G. Wagner, J. Wilzewski, P. Wcisło, S. Yu, E. J. Zak, The HITRAN2016 molecular spectroscopic database, J. Quantitative Spectrosc. Radiative Trans. 203 (2017) 3–69. https://doi.org/10.1016/j.jqsrt.2017.06.038.
R. K. Hanson, R. M. Spearrin, C. S. Goldenstein, Spectroscopy and optical diagnostics for gases, Springer International Publishing AG Switzerland (2016).
C. S. Goldenstein, R. M. Spearrin, J. B. Jeffries, R. K. Hanson, Infrared laser-absorption sensing for combustion gases, Progress energy Combustion Sci. 60 (2017) 132–176. https://doi.org/10.1016/j.pecs.2016.12.002.
K.K. Schwarm, H.Q. Dinh, C.S. Goldenstein, D.I. Pineda, R.M. Spearrin, High-pressure and high-temperature gas cell for absorption spectroscopy studies at wavelengths up to 8 µm. J. Quantitative Spectrosc. Radiative Trans. 227, 145–151 (2019). https://doi.org/10.1016/j.jqsrt.2019.01.029
J. Li, A.P. Nair, K.K. Schwarm, D.I. Pineda, R. Mitchell Spearrin, Temperature-dependent line mixing in the R-branch of the ν3 band of methane. J. Quantitative Spectrosc. Radiative Trans. 255(19), 107271 (2020). https://doi.org/10.1016/j.jqsrt.2020.107271
H. Wang, D. Xie, Q. Chen et al., Kinetic modeling for the deactivation of TiO2 during the photocatalytic removal of low concentration SO2. Chem. Eng. J. 303, 425–432 (2016). https://doi.org/10.1016/J.CEJ.2016.06.015
H. Wang, C. You, Photocatalytic oxidation of SO2 on TiO2 and the catalyst deactivation: a kinetic study. Chem. Eng. J. 350, 268–277 (2018). https://doi.org/10.1016/J.CEJ.2018.05.152
J. Li, K.K. Schwarm, C. Wei et al., Robust cepstral analysis at variable wavelength scan depth for narrowband tunable laser absorption spectroscopy. Measure. Sci. Technol. 32(4), 045502 (2021). https://doi.org/10.1088/1361-6501/abcd6a
H. Xiao, Y. Ru, Q. Cheng et al., Homogeneous and heterogeneous formation of SO3 in flue gases burning high sulfur coal under oxy-fuel combustion conditions. Int. J. Greenhouse Gas Control 78, 420–428 (2018). https://doi.org/10.1016/j.ijggc.2018.09.009
R.F. Majkowski, R.J. Blint, J.C. Hill, Infrared absorption coefficients of gaseous H2SO4 and SO3. Appl. Opt. 17(7), 975–977 (1978). https://doi.org/10.1364/AO.17.000975
M. J. King, W. G. Davenport, M. S. Moats, Oxidation of SO2 to SO3—Equilibrium curves, Sulfuric Acid Manufacture (c) (2013) 125–133. Doi:https://doi.org/10.1016/b978-0-08-098220-5.00010-1.
M. J. King, W. G. Davenport, M. S. Moats, Catalytic oxidation of SO2 to SO3, Sulfuric Acid Manuf. (2013) 73–90. https://doi.org/10.1016/b978-0-08-098220-5.00007-1.
P. Marier, H.P. Dibbs, The catalytic conversion of SO2 to SO3 by fly ash and the capture of SO2 and SO3 by CaO and MgO. Thermochim. Acta 8(1–2), 155–165 (1974). https://doi.org/10.1016/0040-6031(74)85082-3
N.A. Burdett, W.E. Langdon, R.T. Squires, Rate coefficients for the reaction SO2+O2→SO3+O in the temperature range 900–1350 K. J. Inst. Energy 57, 373–376 (1984)
Y. Sarbassov, L. Duan, V. Manovic et al., Sulfur trioxide formation/emissions in coal-fired air-and oxy-fuel combustion processes: a review. Greenhouse Gases 8(3), 402–428 (2018). https://doi.org/10.1002/ghg.1767
D.J. Bayless, J. Jewmaidang, S. Tanneer et al., Kinetics of low-temperature homogeneous SO3 formation for use in flue gas conditioning for improved electrostatic precipitator performance. Proc. Combust. Inst. 28(2), 2499–2505 (2000). https://doi.org/10.1016/S0082-0784(00)80665-7
Acknowledgements
This work was supported by the National Key R&D Program of China (2019YFB2006002), the National Natural Science Foundation of China (11972213, 51906120) and the Tsinghua University-China Huaneng Group Co. Ltd. Joint Institute for Base Energy (HNKJ20-H50).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, J., Ding, Y., Li, Z. et al. Simultaneous measurements of SO2 and SO3 in the heterogeneous conversions of SO2 using QCL absorption spectroscopy. Appl. Phys. B 128, 61 (2022). https://doi.org/10.1007/s00340-022-07776-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00340-022-07776-0