Abstract
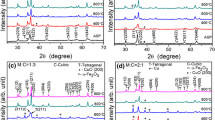
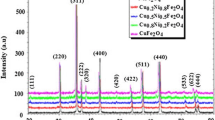
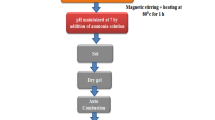
The Nanocrystalline MnFe2O4 ferrite samples have been successfully synthesized by the sol gel method. The amount of citric acid play an important role in the formation of nano particle. The amount of citric acid is minimal, we have achieved a small particles size through this method. It is quite simple and cost-effective. The XRD Patterns confirms the cubic spinel single phase crystal structure. The crystallite size of three samples have been found less than 10 nm. The TEM images show the quantum dot size almost 2 nm which supports the FESEM results. The Energy Dispersive X-ray spectra confirms the appropriate doping in the sample. The Raman spectrum shows existing bands information in the sample. Raman spectrum also confirms the formation of nano particles in the MnFe2O4 ferrite sample. The FTIR spectra of the samples show formation of the nanoparticles. The sample of small particle shows the superparamagnetic behavior at room temperature. Conversely, the larger particle size sample, displayed ferromagnetic behavior at 300 K. Sample lowest particle size sample exhibits a transition in the ZFC mode at 73 K, whereas the highest particle size sample does not show any transition temperature. This behavior indicates that lowest particle size sample contains quantum-dot particles (less then 10 nm), while highest particle size sample exhibits typical of bulk samples behavior. The χ−1-T plots for both samples S1 and S7 are showing a Curie temperature at 73 K.











Similar content being viewed by others
Data availability
No data available.
References
X.-M. Lin, A.C.S. Samia, Synthesis, assembly and physical properties of magnetic nanoparticles. J. Magn. Magn. Mater. 305(1), 100–109 (2006)
M. Suzuki, S.I. Fullem, I.S. Suzuki, L. Wang, C.-J. Zhong, Observation of superspin-glass behavior in Fe 3 O 4 nanoparticles. Phys. Rev. B 79(2), 24418 (2009)
B. Aslibeiki, P. Kameli, H. Salamati, M. Eshraghi, T. Tahmasebi, Superspin glass state in MnFe2O4 nanoparticles. J. Magn. Magn. Mater. 322(19), 2929–2934 (2010). https://doi.org/10.1016/j.jmmm.2010.05.007
S. Khan, M.K. Hossain, 2—classification and properties of nanoparticles, in Woodhead publishing series in composites science and engineering. ed. by S. Mavinkere Rangappa, J. Parameswaranpillai, T.G. Yashas Gowda, S. Siengchin, M.O.B.T.N.B.P.C. Seydibeyoglu (Woodhead Publishing, 2022), pp.15–54. https://doi.org/10.1016/B978-0-12-824272-8.00009-9
Anchal et al., Tailoring quantum dots through citric acid modulation of CoFe2O4 ferrite. Mater. Chem. Phys. 313, 128820 (2024). https://doi.org/10.1016/j.matchemphys.2023.128820
T. Arun et al., Size dependent magnetic and capacitive performance of MnFe2O4 magnetic nanoparticles. Mater. Lett. 276, 128240 (2020). https://doi.org/10.1016/j.matlet.2020.128240
A.K. Singh, O.N. Srivastava, K. Singh, Shape and size-dependent magnetic properties of Fe3O4 nanoparticles synthesized using piperidine. Nanoscale Res. Lett. 12(1), 298 (2017). https://doi.org/10.1186/s11671-017-2039-3
Z. Heydariyan, R. Monsef, M. Salavati-Niasari, Insights into impacts of Co3O4-CeO2 nanocomposites on the electrochemical hydrogen storage performance of g-C3N4: pechini preparation, structural design and comparative study. J. Alloys Compd. 924, 166564 (2022). https://doi.org/10.1016/j.jallcom.2022.166564
S. Zinatloo-Ajabshir, M. Baladi, M. Salavati-Niasari, Enhanced visible-light-driven photocatalytic performance for degradation of organic contaminants using PbWO4 nanostructure fabricated by a new, simple and green sonochemical approach. Ultrason. Sonochem. 72, 105420 (2021). https://doi.org/10.1016/j.ultsonch.2020.105420
S. Mourdikoudis, R.M. Pallares, N.T.K. Thanh, Characterization techniques for nanoparticles: comparison and complementarity upon studying nanoparticle properties. Nanoscale 10(27), 12871–12934 (2018). https://doi.org/10.1039/C8NR02278J
A.G. Díez, M. Rincón-Iglesias, S. Lanceros-Méndez, J. Reguera, E. Lizundia, Multicomponent magnetic nanoparticle engineering: the role of structure-property relationship in advanced applications. Mater. Today Chem. 26, 101220 (2022). https://doi.org/10.1016/j.mtchem.2022.101220
H. Khojasteh, M. Salavati-Niasari, H. Safajou, H. Safardoust-Hojaghan, Facile reduction of graphene using urea in solid phase and surface modification by N-doped graphene quantum dots for adsorption of organic dyes. Diam. Relat. Mater. 79, 133–144 (2017). https://doi.org/10.1016/j.diamond.2017.09.011
K. Vamvakidis, M. Katsikini, D. Sakellari, E.C. Paloura, O. Kalogirou, C. Dendrinou-Samara, Reducing the inversion degree of MnFe2O4 nanoparticles through synthesis to enhance magnetization: evaluation of their 1H NMR relaxation and heating efficiency. Dalt. Trans. 43(33), 12754–12765 (2014). https://doi.org/10.1039/C4DT00162A
M.M. Baig, M.A. Yousuf, P.O. Agboola, M.A. Khan, I. Shakir, M.F. Warsi, Optimization of different wet chemical routes and phase evolution studies of MnFe2O4 nanoparticles. Ceram. Int. 45(10), 12682–12690 (2019). https://doi.org/10.1016/j.ceramint.2019.03.114
L. Nalbandian, A. Delimitis, V.T. Zaspalis, E.A. Deliyanni, D.N. Bakoyannakis, E.N. Peleka, Hydrothermally prepared nanocrystalline Mn–Zn ferrites: synthesis and characterization. Microporous Mesoporous Mater. 114(1–3), 465–473 (2008)
D. Ortega and Q. A. Pankhurst, “Magnetic hyperthermia,” pp. 60–88, 2012, https://doi.org/10.1039/9781849734844-00060
X. Mou, Z. Ali, S. Li, N. He, Applications of magnetic nanoparticles in targeted drug delivery system. J. Nanosci. Nanotechnol. 15(1), 54–62 (2015). https://doi.org/10.1166/jnn.2015.9585
M. Srivastava, B.P. Pandey, N. Mishra, D. Kumar, V.K. Tomar, S. Kumar, Optical properties of 2D pristine and doped Janus WSSe using first-principles study. Nanomater. Energy 11(3–4), 85–91 (2022). https://doi.org/10.1680/jnaen.22.00028
M. Salavati-Niasari, M. Dadkhah, F. Davar, Pure cubic ZrO2 nanoparticles by thermolysis of a new precursor. Polyhedron 28(14), 3005–3009 (2009). https://doi.org/10.1016/j.poly.2009.06.032
S.N. Jaiswal, B.P. Pandey, N. Mishra, D. Kumar, V.K. Tomar, S. Kumar, External electric field impact on electronic properties of CO2-adsorbed 2D MoSe2 monolayer. Pramana 97(3), 140 (2023). https://doi.org/10.1007/s12043-023-02613-1
G. Song et al., Carbon-coated FeCo nanoparticles as sensitive magnetic-particle-imaging tracers with photothermal and magnetothermal properties. Nat. Biomed. Eng. 4(3), 325–334 (2020). https://doi.org/10.1038/s41551-019-0506-0
G. Singh et al., Synthesis of gadolinium oxide nanodisks and gadolinium doped iron oxide nanoparticles for MR contrast agents. J. Mater. Chem. B 5(3), 418–422 (2017). https://doi.org/10.1039/C6TB02854C
B.L. Choudhary, N. Kumari, J. Kumari, A. Kumar, S.N. Dolia, Relaxation mechanism in Ni0.5Zn0.5Fe2O4 nanocrystalline ferrite at a lower temperature. Mater. Lett. 304, 130731 (2021). https://doi.org/10.1016/j.matlet.2021.130731
B.L. Choudhary et al., Low temperature field dependent magnetic study of the Zn0. 5Co0. 5Fe2O4 nanoparticles. J. Magn. Magn. Mater. 536, 168102 (2021). https://doi.org/10.1016/j.jmmm.2021.168102
M. Salvador, A. Moyano, J.C. Martínez-García, M.C. Blanco-López, M. Rivas, Synthesis of superparamagnetic iron oxide nanoparticles: SWOT analysis towards their conjugation to biomolecules for molecular recognition applications. J. Nanosci. Nanotechnol. 19(8), 4839–4856 (2019). https://doi.org/10.1166/jnn.2019.16931
V. Mameli, M.S. Angotzi, C. Cara, C. Cannas, Liquid phase synthesis of nanostructured spinel ferrites-a review. J. Nanosci. Nanotechnol. 19(8), 4857–4887 (2019). https://doi.org/10.1166/jnn.2019.16808
W. Wu, Z. Wu, T. Yu, C. Jiang, W.-S. Kim, Recent progress on magnetic iron oxide nanoparticles: synthesis, surface functional strategies and biomedical applications. Sci. Technol. Adv. Mater. 16(2), 23501 (2015). https://doi.org/10.1088/1468-6996/16/2/023501
V. Dhayal, M.K. Atal, B.L. Choudhary, M. Nagar, R. Bohra, Glycol modified titanosiloxane as molecular precursor for homogenous titania-silica material: Synthesis and characterization. J. Sol-Gel Sci. Technol. (2009). https://doi.org/10.1007/s10971-009-2008-0
B.L. Choudhary et al., Exploring magnetic behaviour in La0.70Pr0.30Mn0.8Co0.2O3 perovskite. J. Supercond. Nov. Magn. (2022). https://doi.org/10.1007/s10948-022-06173-0
V. Dhayal et al., Molecular precursors for the preparation of homogenous zirconia-silica materials by hydrolytic sol-gel process in organic media. Crystal structures of [Zr{OSi(OtBu)3}4(H2O)2]·2H2O and [Ti(OtBu){OSi(OtBu)3}3]. Dalt. Trans. (2012). https://doi.org/10.1039/c2dt12127a
A.R. Sanwaria et al., Sol-gel synthesis of highly pure α-Al2O3 nano-rods from a new class of precursors of salicylaldehyde-modified aluminum(iii) isopropoxide. Crystal and molecular structure of [Al(OC 6H4CHO)3]. RSC Adv. 4(57), 30081–30089 (2014). https://doi.org/10.1039/c4ra03245d
M. Panahi-Kalamuei, M. Salavati-Niasari, S.M. Hosseinpour-Mashkani, Facile microwave synthesis, characterization, and solar cell application of selenium nanoparticles. J. Alloys Compd. 617, 627–632 (2014). https://doi.org/10.1016/j.jallcom.2014.07.174
A. Omelyanchik et al., Effect of citric acid on the morpho-structural and magnetic properties of ultrasmall iron oxide nanoparticles. J. Alloys Compd. 883, 160779 (2021). https://doi.org/10.1016/j.jallcom.2021.160779
G. Lal et al., Exploring the structural, elastic, optical, dielectric and magnetic characteristics of Ca2+ incorporated superparamagnetic Zn0.5−xCa0.1Co0.4+xFe2O4 (x = 0.0, 0.05 & 0.1) nanoferrites. J. Alloys Compd. 886, 161190 (2021). https://doi.org/10.1016/j.jallcom.2021.161190
B.L. Choudhary et al., Irreversible magnetic behavior with temperature variation of Ni0.5Co0.5Fe2O4 nanoparticles. J. Magn. Magn. Mater. (2020). https://doi.org/10.1016/j.jmmm.2020.166861
A. Kumar, M.K. Gora, S. Kumar, B.L. Choudhary, P.K. Maheshwari, S.N. Dolia, Reitveld refinement, structural, optical band gap and low-temperature magnetic characterization of Gd3+ doped spinel cubic CoFe2O4 nanoparticles. Bangladesh J. Sci. Ind. Res. 57(3), 173–186 (2022). https://doi.org/10.3329/bjsir.v57i3.62019
K. Kalantari, M.B. Ahmad, H.R. Fard Masoumi, K. Shameli, M. Basri, R. Khandanlou, Rapid and high capacity adsorption of heavy metals by Fe3O4/montmorillonite nanocomposite using response surface methodology: Preparation, characterization, optimization, equilibrium isotherms, and adsorption kinetics study. J. Taiwan Inst. Chem. Eng. 49, 192–198 (2015). https://doi.org/10.1016/j.jtice.2014.10.025
N.M. Mahmoodi, Magnetic ferrite nanoparticle–alginate composite: synthesis, characterization and binary system dye removal. J. Taiwan Inst. Chem. Eng. 44(2), 322–330 (2013). https://doi.org/10.1016/j.jtice.2012.11.014
M. Amiri, M. Salavati-Niasari, A. Akbari, T. Gholami, Removal of malachite green (a toxic dye) from water by cobalt ferrite silica magnetic nanocomposite: Herbal and green sol-gel autocombustion synthesis. Int. J. Hydrogen Energy 42(39), 24846–24860 (2017). https://doi.org/10.1016/j.ijhydene.2017.08.077
M.S. Rulaniya et al., Temperature-dependent magnetic and electrical behavior in the La0.50Pr0.50Mn0.8Co0.2O3 perovskite. Emergent Mater. (2023). https://doi.org/10.1007/s42247-023-00536-6
B.L. Choudhary et al., Ferro- to paramagnetic phase transition in La0.90Pr0.10Mn0.8Co0.2O3 perovskite. J. Low Temp. Phys. 210(1), 271–284 (2023). https://doi.org/10.1007/s10909-022-02930-6
B.L. Choudhary et al., Oxygen vacancy induced structural and domain size-controlled magnetic behavior of La0.67Ca0.33MnO3 perovskite. J. Mater. Sci. Mater. Electron. 33(9), 6829–6841 (2022). https://doi.org/10.1007/s10854-022-07861-2
A. Ravina, S. Dalela, S. Kumar, B.L. Choudhary, P.A. Alvi, Structural, optical and Raman studies of Co3O4 nano-particles. Mater. Today Proc. (2022). https://doi.org/10.1016/j.matpr.2022.08.513
B. L. Choudhary, A. Krishnamurthy, and B. K. Srivastava, “Size dependence of magneto-resistive behaviour of nano-particle magnetite,” Indian J. Pure Appl. Phys., 49(3) (2011).
A. Kumar et al., Impact of Gd3+ doping on structural, electronic, magnetic, and photocatalytic properties of MnFe2O4 nanoferrites and application in dye-polluted wastewater remediation. Environ. Sci. Pollut. Res. (2022). https://doi.org/10.1007/s11356-022-23420-y
N. Kumari et al., Structural and magnetic behavior in nanocrystalline Ni0.5Co0.5Fe2O4. Mater. Today Proc. (2022). https://doi.org/10.1016/j.matpr.2022.08.241
G. Lal, K. Punia, S.N. Dolia, P.A. Alvi, B.L. Choudhary, S. Kumar, Structural, cation distribution, optical and magnetic properties of quaternary Co0.4+xZn0.6-xFe2O4 (x = 0.0, 0.1 and 0.2) and Li doped quinary Co0.4+xZn0.5-xLi0.1Fe2O4 (x = 0.0, 0.05 and 0.1) nanoferrites. J. Alloys Compd. 828, 154388 (2020). https://doi.org/10.1016/j.jallcom.2020.154388
A. Kumar, M.K. Gora, S. Kumar, B.L. Choudhary, R.K. Singhal, S.N. Dolia, Study of electronic structure and dielectric properties of Gd-doped cobalt nanoferrites. J. Korean Phys. Soc. 81(9), 894–902 (2022). https://doi.org/10.1007/s40042-022-00612-w
H. Cheema, S. Kumar, P.A. Alvi, B.L. Choudhary, U. Kumar, Synthesis and physical properties of nanopowder and electrical properties of bulk samples of ZnFe2-xNixO4 (x: 0, 0.05, 0.10). Adv. Powder Technol. 31(10), 4241–4252 (2020). https://doi.org/10.1016/j.apt.2020.09.001
C. Simon et al., Magnetic properties and structural analysis on spinel MnFe2O4 nanoparticles prepared via non-aqueous microwave synthesis. Zeitschrift für Anorg. und Allg. Chemie 647(22), 2061–2072 (2021). https://doi.org/10.1002/zaac.202100190
G. Srivastava, S. Dalela, S. Kumar, B.L. Choudhary, P.A. Alvi, Structural and Raman studies of MnO2 and Mn2O3 nano-particles. Mater. Today Proc. (2022). https://doi.org/10.1016/j.matpr.2022.08.515
S. Sharifi, K. Rahimi, A. Yazdani, Highly improved supercapacitance properties of MnFe2O4 nanoparticles by MoS2 nanosheets. Sci. Rep. 11(1), 8378 (2021). https://doi.org/10.1038/s41598-021-87823-6
W. Wang et al., Microstructure and magnetic properties of MFe2O4 (M = Co, Ni, and Mn) ferrite nanocrystals prepared using colloid mill and hydrothermal method. J. Appl. Phys. 117(17), 17A328 (2015). https://doi.org/10.1063/1.4917463
K.K. Khichar et al., Structural, optical, and surface morphological studies of ethyl cellulose/graphene oxide nanocomposites. Polym. Compos. (2020). https://doi.org/10.1002/pc.25576
C.N. Chinnasamy et al., Size dependent magnetic properties and cation inversion in chemically synthesized MnFe2O4 nanoparticles. J. Appl. Phys. 101(9), 09M509 (2007). https://doi.org/10.1063/1.2710218
G. Balaji, N.S. Gajbhiye, G. Wilde, J. Weissmüller, Magnetic properties of MnFe2O4 nanoparticles. J. Magn. Magn. Mater. 242–245, 617–620 (2002). https://doi.org/10.1016/S0304-8853(01)01043-5
O. Mounkachi, R. Lamouri, E. Salmani, M. Hamedoun, A. Benyoussef, H. Ez-Zahraouy, Origin of the magnetic properties of MnFe2O4 spinel ferrite: Ab initio and Monte Carlo simulation. J. Magn. Magn. Mater. 533, 168016 (2021). https://doi.org/10.1016/j.jmmm.2021.168016
B. Aslibeiki, P. Kameli, M.H. Ehsani, MnFe2O4 bulk, nanoparticles and film: a comparative study of structural and magnetic properties. Ceram. Int. 42(11), 12789–12795 (2016). https://doi.org/10.1016/j.ceramint.2016.05.041
R. Singh Yadav et al., Impact of sonochemical synthesis condition on the structural and physical properties of MnFe2O4 spinel ferrite nanoparticles. Ultrason. Sonochem. 61, 104839 (2020). https://doi.org/10.1016/j.ultsonch.2019.104839
B.L. Choudhary, S. Kumar, A. Krishnamurthy, B.K. Srivastava, Magnetic behaviour of praseodymium substituted perovskites La 1-xPrxMn0.8Co0.2O3. AIP Conf. Proc. 1536, 991–992 (2013). https://doi.org/10.1063/1.4810565
B.L. Choudhary, A. Krishnamurthy, B.K. Srivastava, Magnetic behaviour of bismuth substituted perovskites La 1-xBixMn0.8Co0.2O3. AIP Conf. Proc. 1313, 307–309 (2010). https://doi.org/10.1063/1.3530526
G. Lal et al., Wasp-waisted like magnetic behavior of nanocrystalline CoFe2O4 at 5K. in AIP Conference Proceedings, 2020, vol. 2220. https://doi.org/10.1063/5.0001729
Acknowledgements
The authors would like to express their appreciation to the Department of Science and Technology (DST), Government of India, New Delhi, for providing essential facilities to Banasthali Vidyapith under the CURIE project. Additionally, K.K. Palsaniya, M.S. Rulaniya, and Priya acknowledge the University Grants Commission, New Delhi, for their fellowship support. S.R. Choudhary, P.M. Saini and R.K. Beniwal acknowledge the Council of Scientific and Industrial Research (CSIR), New Delhi, Government of India, for their fellowships.
Author information
Authors and Affiliations
Contributions
Namita Kumari: Writing original draft, methodology, Sarita: investigation, data analysis, Anchal: XRD data analysis & investigation, Priya: SEM Measurements, K. K. Palsaniya: Investigation and FTIR measurements, R. K. Beniwal: FTIR analysis, S. R. Choudhary: X-ray measurements, M.S. Rulaniya: FESEM Analysis, P. M. Saini:FTIR analysis, S. N. Dolia: Data Analysis, P. A. Alvi: Review & editing, B. L. Choudhary: Conceptualization, Data analysis, Supervision.
Corresponding author
Ethics declarations
Conflict of interest
Authors of this manuscript declared no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kumari, N., Sarita, Anchal et al. The role of citric acid for formation of nanocrystalline MnFe2O4 ferrite. Appl. Phys. A 130, 266 (2024). https://doi.org/10.1007/s00339-024-07423-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-024-07423-9