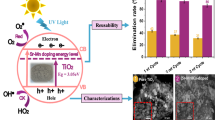
Abstract
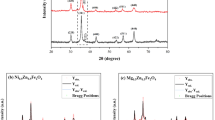
Magnesium spinel ferrite (MgFe2O4) and cerium-doped magnesium spinel ferrite (MgFe2-xCexO4) (x = 0.0, 0.05, 0.1) were synthesized using an inexpensive coprecipitation method. The investigation focused on the influence of cerium on the physicochemical properties and the photocatalytic activity of this material under sunlight irradiation. The various characterization techniques, including X-ray powder diffraction (XRD), Fourier transform infrared spectroscopy (FT-IR), environmental scanning electron microscopy (ESEM-EDX), the Brunauer–Emmett–Teller (BET) method, and vibrating sample magnetometer (VSM), were used to determine the structural, morphological, and magnetic properties of the prepared samples. The increase in cerium percentage was found to better agglomerate the sheet-like morphology formed in the pure spinel and increase the average crystallite size, with a decrease in the magnetic properties. The optical band gap measured by UV–visible spectroscopy decreased from 2.21 eV, 2.21 eV, and 2.36 eV, accompanied by a change in the specific surface area of 47.23 m2/g, 57.86 m2/g, and 47.25 m2/g for MgFe2O4, MgFe1.95Ce0.05O4, and MgFe1.90Ce0.1O4, respectively. The maximum photocatalytic degradation of methylene blue under sunlight irradiation was achieved for the MgFe1.95Ce0.05O4 (58.35%) in the absence of hydrogen peroxide, and the maximum degradation for Fenton-like processes was achieved in the presence of MgFe1.90Ce0.1O4 (91.05%) within 180 min.












Similar content being viewed by others
Data availability
The data that support the findings of this study are available on request from the corresponding author Hebbaz Abdennour.
References
M.M. Vopson, Fundamentals of multiferroic materials and their possible applications. Crit. Rev. Solid State Mater. Sci. 40(4), 223–250 (2015). https://doi.org/10.1080/10408436.2014.992584
A. Sasmal, A.K. Nayak, Morphology-dependent solvothermal synthesis of spinel NiCo2O4 nanostructures for enhanced energy storage device application. J. Energy Storage 58, 106342 (2023). https://doi.org/10.1016/j.est.2022.106342
Q. Mahmood et al., Room temperature ferromagnetism and thermoelectric behavior of calcium based spinel chalcogenides CaZ2S4 (Z = Ti, V, Cr, Fe) for spintronic applications. J. Phys. Chem. Solids 167, 110742 (2022). https://doi.org/10.1016/j.jpcs.2022.110742
P.L. Mahapatra, S. Das, N.A. Keasberry, S.B. Ibrahim, D. Saha, Copper ferrite inverse spinel-based highly sensitive and selective chemiresistive gas sensor for the detection of formalin adulteration in fish. J. Alloys Compd. 960, 170792 (2023). https://doi.org/10.1016/j.jallcom.2023.170792
A. Manikandan, M. Durka, K. Seevakan, S.A. Antony, A Novel One-Pot Combustion Synthesis and Opto-magnetic Properties of Magnetically Separable Spinel Mn x Mg 1–x Fe2 O 4(0.0 ≤ x ≤ 0.5) Nanophotocatalysts. J. Supercond. Magn. 28(4), 1405–1416 (2015). https://doi.org/10.1007/s10948-014-2864-x
A. Manohar et al., Investigation on the physico-chemical properties, hyperthermia and cytotoxicity study of magnesium doped manganese ferrite nanoparticles. Mater. Chem. Phys. 287, 126295 (2022). https://doi.org/10.1016/j.matchemphys.2022.126295
K.M. Batoo et al., Improved microwave absorption and EMI shielding properties of Ba-doped Co–Zn ferrite. Ceram. Int. 48(3), 3328–3343 (2022). https://doi.org/10.1016/j.ceramint.2021.10.108
V.K. Chakradhary, A. Ansari, M.J. Akhtar, Design, synthesis, and testing of high coercivity cobalt doped nickel ferrite nanoparticles for magnetic applications. J. Magn. Magn. Mater. 469, 674–680 (2019). https://doi.org/10.1016/j.jmmm.2018.09.021
Y. Slimani et al., Calcination effect on the magneto-optical properties of vanadium substituted NiFe2O4 nanoferrites. J. Mater. Sci. Mater. Electron. 30(10), 9143–9154 (2019). https://doi.org/10.1007/s10854-019-01243-x
S. K. Sharma, Ed., Spinel Nanoferrites: Synthesis, Properties and Applications. in Topics in Mining, Metallurgy and Materials Engineering. Cham: Springer International Publishing, 2021. doi: https://doi.org/10.1007/978-3-030-79960-1.
Q. Chen, A.J. Rondinone, B.C. Chakoumakos, Z.J. Zhang, Synthesis of superparamagnetic MgFe2O4 nanoparticles by coprecipitation. J. Magn. Magn. Mater. 194(1–3), 1–7 (1999)
J. Kurian, M.J. Mathew, Structural, optical and magnetic studies of CuFe2O4, MgFe2O4 and ZnFe2O4 nanoparticles prepared by hydrothermal/solvothermal method. J. Magn. Magn. Mater. 451, 121–130 (2018). https://doi.org/10.1016/j.jmmm.2017.10.124
Y. Ichiyanagi, M. Kubota, S. Moritake, Y. Kanazawa, T. Yamada, T. Uehashi, Magnetic properties of Mg-ferrite nanoparticles. J. Magn. Magn. Mater. 310(2), 2378–2380 (2007). https://doi.org/10.1016/j.jmmm.2006.10.737
K. Mukherjee, D.C. Bharti, S.B. Majumder, Solution synthesis and kinetic analyses of the gas sensing characteristics of magnesium ferrite particles. Sens. Actuators B Chem. 146(1), 91–97 (2010). https://doi.org/10.1016/j.snb.2010.02.020
N. Sivakumar et al., Nanostructured MgFe2O4 as anode materials for lithium-ion batteries. J. Alloys Compd. 509(25), 7038–7041 (2011). https://doi.org/10.1016/j.jallcom.2011.03.123
J.M. Poyatos, M.M. Muñio, M.C. Almecija, J.C. Torres, E. Hontoria, F. Osorio, Advanced oxidation processes for wastewater treatment: state of the art. Water Air Soil Pollut. 205(1–4), 187–204 (2010). https://doi.org/10.1007/s11270-009-0065-1
J.-M. Herrmann, C. Guillard, P. Pichat, Heterogeneous photocatalysis : an emerging technology for water treatment. Catal. Today 17(1–2), 7–20 (1993). https://doi.org/10.1016/0920-5861(93)80003-J
L. Zhao et al., Synthesis, characterization and adsorptive performance of MgFe2O4 nanospheres for SO2 removal. J. Hazard. Mater. 184(1–3), 704–709 (2010). https://doi.org/10.1016/j.jhazmat.2010.08.096
M. Israr, J. Iqbal, A. Arshad, P. Gómez-Romero, R. Benages, Multifunctional MgFe2O4/GNPs nanocomposite: graphene-promoted visible light driven photocatalytic activity and electrochemical performance of MgFe2O4 nanoparticles. Solid State Sci. 110, 106363 (2020). https://doi.org/10.1016/j.solidstatesciences.2020.106363
L. Lu, J. Li, J. Yu, P. Song, D.H.L. Ng, A hierarchically porous MgFe2O4/γ-Fe2O3 magnetic microspheres for efficient removals of dye and pharmaceutical from water. Chem. Eng. J. 283, 524–534 (2016). https://doi.org/10.1016/j.cej.2015.07.081
A. F. Cabrera, C. E. Rodríguez Torres, S. G. Marchetti, and S. J. Stewart, “Degradation of methylene blue dye under dark and visible light conditions in presence of hybrid composites of nanostructured MgFe2O4 ferrites and oxygenated organic compounds,” J. Environ. Chem. Eng., vol. 8, no. 5, p. 104274, 2020, doi: https://doi.org/10.1016/j.jece.2020.104274.
T.A. Nguyen, R.-S. Juang, Treatment of waters and wastewaters containing sulfur dyes: a review. Chem. Eng. J. 219, 109–117 (2013). https://doi.org/10.1016/j.cej.2012.12.102
T. Platzek, Overview on toxicity and exposure to azo dyes and aromatic amines. Toxicol. Lett. 221, S53 (2013). https://doi.org/10.1016/j.toxlet.2013.06.193
S. Haider, S.S. Shar, I. Shakir, P.O. Agboola, Visible light active Cu-doped iron oxide for photocatalytic treatment of methylene blue. Ceram. Int. 48(6), 7605–7612 (2022). https://doi.org/10.1016/j.ceramint.2021.11.304
W.S. Kuo, P.H. Ho, Solar photocatalytic decolorization of methylene blue in water. Chemosphere 45(1), 77–83 (2001). https://doi.org/10.1016/S0045-6535(01)00008-X
M. Rojas-Cervantes, E. Castillejos, Perovskites as catalysts in advanced oxidation processes for wastewater treatment. Catalysts 9(3), 230 (2019). https://doi.org/10.3390/catal9030230
B. Elvers, G. Bellussi (eds.), Ullmann’s encyclopedia of industrial chemistry, 7th, completely rev, ed. (Wiley, Weinheim, 2011)
S.N.E. Hassan, T.M. Hussein, M. El, Eldeeb, “Photodynamic therapy using methylene blue and intense pulsed light versus intense pulsed light alone in treatment of verruca: a randomized controlled study.” Photodiagnosis Photodyn. Ther. 36, 102541 (2021). https://doi.org/10.1016/j.pdpdt.2021.102541
P. Rajasulochana, V. Preethy, Comparison on efficiency of various techniques in treatment of waste and sewage water – A comprehensive review. Resour.-Effic. Technol. 2(4), 175–184 (2016). https://doi.org/10.1016/j.reffit.2016.09.004
T. Baysal, N. Noor, A. Demir, Nanofibrous MgO composites: structures, properties, and applications. Polym.-Plast. Technol. Mater. 59(14), 1522–1551 (2020). https://doi.org/10.1080/25740881.2020.1759212
A. Goktas, S. Modanlı, A. Tumbul, A. Kilic, Facile synthesis and characterization of ZnO, ZnO:Co, and ZnO/ZnO: Co nano rod-like homojunction thin films: Role of crystallite/grain size and microstrain in photocatalytic performance. J. Alloys Compd. 893, 162334 (2022). https://doi.org/10.1016/j.jallcom.2021.162334
D.A. Salinas, C.L. Marchena, L.B. Pierella, G. Pecchi, Catalytic oxidation of 2-(methylthio)-benzothiazole on alkaline earth titanates, ATiO3 (A = Ca, Sr, Ba). Mol. Catal. 438, 76–85 (2017). https://doi.org/10.1016/j.mcat.2017.05.019
L. Mi et al., Synthesis of BaTiO3 nanoparticles by sol-gel assisted solid phase method and its formation mechanism and photocatalytic activity. Ceram. Int. 46(8), 10619–10633 (2020). https://doi.org/10.1016/j.ceramint.2020.01.066
K. Shetty et al., A comparative study on CuFe 2 O 4, ZnFe 2 O 4 and NiFe 2 O 4: morphology, Impedance and Photocatalytic studies. Mater. Today Proc. 4(11), 11806–11815 (2017). https://doi.org/10.1016/j.matpr.2017.09.098
A. Charles et al., Facile synthesis of CaFe2O4 for visible light driven treatment of polluting palm oil mill effluent: photokinetic and scavenging study. Sci. Total. Environ. 661, 522–530 (2019). https://doi.org/10.1016/j.scitotenv.2019.01.195
M. George et al., Evaluation of Cu–MgFe2O4 spinel nanoparticles for photocatalytic and antimicrobial activates. J. Phys. Chem. Solids 153, 110010 (2021). https://doi.org/10.1016/j.jpcs.2021.110010
T. Wu, G. Shen, F. Ding, D. Li, H. Zhong, Effects of La3+ doping on the structure, properties and application of Mn–Zn ferrite. Mater. Chem. Phys. 252, 123328 (2020). https://doi.org/10.1016/j.matchemphys.2020.123328
S.P. Keerthana, R. Yuvakkumar, P.S. Kumar, G. Ravi, D. Velauthapillai, Rare earth metal (Sm) doped zinc ferrite (ZnFe2O4) for improved photocatalytic elimination of toxic dye from aquatic system. Environ. Res. 197, 111047 (2021). https://doi.org/10.1016/j.envres.2021.111047
M. Dhiman, B. Chudasama, V. Kumar, K.B. Tikoo, S. Singhal, Augmenting the photocatalytic performance of cobalt ferrite via change in structural and optical properties with the introduction of different rare earth metal ions. Ceram. Int. 45(3), 3698–3709 (2019). https://doi.org/10.1016/j.ceramint.2018.11.033
K. Elayakumar et al., Enhanced magnetic property and antibacterial biomedical activity of Ce3+ doped CuFe2O4 spinel nanoparticles synthesized by sol-gel method. J. Magn. Magn. Mater. 478, 140–147 (2019). https://doi.org/10.1016/j.jmmm.2019.01.108
J. Hou et al., One-pot hydrothermal synthesis of CdS–CuS decorated TiO2 NTs for improved photocatalytic dye degradation and hydrogen production. Ceram. Int. 47(21), 30860–30868 (2021). https://doi.org/10.1016/j.ceramint.2021.07.268
K. Shetty et al., Designing MgFe2O4 decorated on green mediated reduced graphene oxide sheets showing photocatalytic performance and luminescence property. Phys. B Condens. Matter 507, 67–75 (2017). https://doi.org/10.1016/j.physb.2016.11.021
J.Y. Patil, D.Y. Nadargi, I.S. Mulla, S.S. Suryavanshi, Cerium doped MgFe2O4 nanocomposites: highly sensitive and fast response-recoverable acetone gas sensor. Heliyon 5(6), e01489 (2019). https://doi.org/10.1016/j.heliyon.2019.e01489
T. Zhou et al., Efficient degradation of rhodamine B with magnetically separable Ag3PO4@MgFe2O4 composites under visible irradiation. J. Alloys Compd. 735, 1277–1290 (2018). https://doi.org/10.1016/j.jallcom.2017.11.245
Y. Liu, J. Wang, Multivalent metal catalysts in Fenton/Fenton-like oxidation system: a critical review. Chem. Eng. J. 466, 143147 (2023). https://doi.org/10.1016/j.cej.2023.143147
L. Sharma, R. Kakkar, Magnetically retrievable one-pot fabrication of mesoporous magnesium ferrite (MgFe2O4) for the remediation of chlorpyrifos and real pesticide wastewater. J. Environ. Chem. Eng. 6(6), 6891–6903 (2018). https://doi.org/10.1016/j.jece.2018.10.058
F. Andish-Lifshagerd, A. Habibi-Yangjeh, M. Habibi, Y. Akinay, Facile synthesis of ZnO/CeO2/CeFeO3 photocatalysts sensitive to visible light with tandem n-n heterojunctions and improved performance towards tetracycline and dye effluent removals. J. Photochem. Photobiol. Chem. 448, 115351 (2024). https://doi.org/10.1016/j.jphotochem.2023.115351
S. Dadras, S. Falahati, S. Dehghani, Effects of graphene oxide doping on the structural and superconducting properties of YBa2Cu3O7−δ. Phys. C Supercond. Appl. 548, 65–67 (2018). https://doi.org/10.1016/j.physc.2018.02.010
S. Gaba, A. Kumar, P.S. Rana, Influence of Ce3+ ion doping on structural and magnetic properties of magnesium nanoferrite. J. Supercond. Magn. 32(5), 1465–1474 (2019). https://doi.org/10.1007/s10948-018-4846-x
T. Kousar et al., Wet-chemical synthesis of nanostructured Ce-doped mixed metal ferrites for the effective removal of azo dyes from industrial discharges. Ceram. Int. 48(8), 11858–11868 (2022). https://doi.org/10.1016/j.ceramint.2022.01.057
S. Patil et al., Magnetic Eu-doped MgFe2O4 nanomaterials: an investigation of their structural, optical and enhanced visible-light-driven photocatalytic performance. Environ. Nanotechnol. Monit. Manag. 13, 100268 (2020). https://doi.org/10.1016/j.enmm.2019.100268
D. Alhashmialameer et al., Copper-doped magnesium ferrite and its composite with rGO: synthesis, characterization, and degradation of organic effluents and antibacterial study. Ceram. Int. 48(16), 24100–24113 (2022). https://doi.org/10.1016/j.ceramint.2022.05.373
J.B. Condon, Surface area and porosity determinations by physisorption: measurements and theory, 1st edn. (Elsevier, Amsterdam; Boston, 2006)
Z. Hammache, A. Soukeur, S. Omeiri, B. Bellal, M. Trari, Physical and photo-electrochemical properties of MgFe2O4 prepared by sol gel route: application to the photodegradation of methylene blue. J. Mater. Sci. Mater. Electron. 30(6), 5375–5382 (2019). https://doi.org/10.1007/s10854-019-00830-2
I.A. Parray, A. Somvanshi, S.A. Ali, Study of microstructural, ferroelectric and magnetic properties of cerium substituted magnesium ferrite and its potential application as hydroelectric cell. Ceram. Int. 49(4), 6946–6957 (2023). https://doi.org/10.1016/j.ceramint.2022.10.223
A. Sundaresan, R. Bhargavi, N. Rangarajan, U. Siddesh, C.N.R. Rao, Ferromagnetism as a universal feature of nanoparticles of the otherwise nonmagnetic oxides. Phys. Rev. B 74(16), 161306 (2006). https://doi.org/10.1103/PhysRevB.74.161306
Y. Liu, Z. Lockman, A. Aziz, J. MacManus-Driscoll, Size dependent ferromagnetism in cerium oxide (CeO 2) nanostructures independent of oxygen vacancies. J. Phys. Condens. Matter 20(16), 165201 (2008). https://doi.org/10.1088/0953-8984/20/16/165201
C. Sedrati, S. Alleg, H. Boussafel, and A. Bendali Hacine, “Structure and magnetic properties of nickel ferrites synthesized by a facile co-precipitation method: effect of the Fe/Ni ratio,” J. Mater. Sci. Mater. Electron., vol. 32, no. 19, pp. 24548–24559, 2021, doi: https://doi.org/10.1007/s10854-021-06932-0.
M. Fallot, Ferromagnétisme des alliages de fer. Ann. Phys. 11(6), 305–387 (1936). https://doi.org/10.1051/anphys/193611060305
Y. Liu, D. Shindō, and D. J. Sellmyer, Eds., Handbook of advanced magnetic materials. New York : [Beijing]: Springer ; Tsinghua University Press, 2006.
A. Ivanets et al., Effect of metal ions adsorption on the efficiency of methylene blue degradation onto MgFe2O4 as Fenton-like catalysts. Colloids Surf. Physicochem. Eng. Asp. 571, 17–26 (2019). https://doi.org/10.1016/j.colsurfa.2019.03.071
A. Manohar, V. Vijayakanth, M.R. Pallavolu, K.H. Kim, Effects of Ni - substitution on structural, magnetic hyperthermia, photocatalytic and cytotoxicity study of MgFe2O4 nanoparticles. J. Alloys Compd. 879, 160515 (2021). https://doi.org/10.1016/j.jallcom.2021.160515
Author information
Authors and Affiliations
Contributions
All the authors contributed to the study conception and design. Investigation, visualization, project administration, and writing were performed by Hebbaz Abdennour. Investigation, visualization, project administration, and correcting were performed by Belhani Mehdi. Review, and supervision were performed by Tahraoui Tarek. The first draft of the manuscript was written by Hebbaz Abdennour. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose. The authors have no competing interests to declare that are relevant to the content of this article. All the authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript. The authors have no financial or proprietary interests in any material discussed in this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hebbaz, A., Belhani, M. & Tahraoui, T. Synthesis of cerium-doped magnesium spinel ferrites and study of their physical properties for photocatalytic applications under sunlight irradiation. Appl. Phys. A 130, 94 (2024). https://doi.org/10.1007/s00339-023-07219-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-023-07219-3